RBSE Solutions for Class 10 Science Chapter 8 Carbon and its Compounds are part of RBSE Solutions for Class 10 Science. Here we have given Rajasthan Board RBSE Class 10 Science Solutions Chapter 8 Carbon and its Compounds.
| Board | RBSE |
| Textbook | SIERT, Rajasthan |
| Class | Class 10 |
| Subject | Science |
| Chapter | Chapter 8 |
| Chapter Name | Carbon and its Compounds |
| Number of Questions Solved | 97 |
| Category | RBSE Solutions |
Rajasthan Board RBSE Class 10 Science Solutions Chapter 8 Carbon and its Compounds
Textbook Questions Solved
I. Multiple Choice Questions:
Question 1:
What is the bond angle in (RBSESolutions.com) methane?
(a) 109°28′
(b) 120°
(c) 180°
(d) 105°
Answer:
(a) 109°28′
Question 2:
C5H10 is the formula of which hydrocarbon?
(a) Pentane
(b) Pentene
(c) Pentyne
(d) Pentadiene
Answer:
(b) Pentene
![]()
Question 3:
What is the formula of (RBSESolutions.com) Freon-11?
(a) CFCl3
(b) C2F2Cl4
(c) CF2Cl2
(d) C2F4Cl
Answer:
(a) CFCl3
Question 4:
Natural rubber is a polymer of which substance?
(a) Neoprene
(b) Isoprene
(c) 1,3-Butadiene
(d) Buna-N
Answer:
(b) Isoprene
Question 5:
Which allotrope of carbon is a good conductor (RBSESolutions.com) of electricity?
(a) Diamond
(b) Graphite
(c) Fullerene
(d) Coke
Answer:
(b) Graphite
Question 6:
For improving the tensile strength and quality of natural rubber, it is heated with sulphur. What is the name of this process?
(a) Polymerization
(b) Saponification
(c) Vulcanisation
(d) Equalization
Answer:
(c) Vulcanisation
Question 7:
What is the prefix if number of carbon (RBSESolutions.com) atoms is 3?
(a) Eth-
(b) Prop-
(c) But-
(d) Pent-
Answer:
(b) Prop-
Question 8:
What is the IUPAC name of CH2 = CH-CH2 -Cl?
(a) 1 – Chloro – 2 – propene
(b) Prop – 1 – chloro – 2 – ene
(c) 3 – Chloro – 2 – propene
(d) 3 – Chloro – 1 – propene
Answer:
(d) 3 – Chloro – 1 – propene
![]()
Carbon and its Compounds Very Short Answer Type Questions
Question 9:
Write the general for mulae of alkane, (RBSESolutions.com) alkene and alkyne.
Answer:
Alkane: CnH2n+2
Alkene: CnH2n
Alkyne: CnH2n-2
Question 10:
Hydrocarbons are made up of which elements?
Answer:
Hydrogen and carbon
Question 11:
Write the full form of IUPAC.
Answer:
International Union of Pure & Applied Chemistry
Question 12:
Define vulcanization.
Answer:
The process of improving tensile strength and (RBSESolutions.com) quality of natural rubber by heating it with sulphur is called vulcanization.
Question 13:
What could be the total number of carbon atoms in fullerene?
Answer:
Number of carbon atoms in fullerene can be 60, 70 or more.
Question 14:
What is the geometry of carbon atom?
Answer:
Carbon is a tetrahedron in which four valencies are towards four angles of tetrahedron. Carbon atom is at the centre of tetrahedron. The bond angle between each valency is 109°28′.
Question 15:
What is Freon.
Answer:
When chlorine and fluorine make compounds with carbon, the compounds are called chlorofluorocarbons,.Pqly-chlprp-flupro alkane,is called Freon.
![]()
Question 16:
Which scientist was the first to synthesize (RBSESolutions.com) organic compound?
Answer:
Friedrich Wohler
Question 17:
Write the full form of CNG.
Answer:
Compressed Natural Gas
Question 18:
Orion is made by polymerization of which molecule?
Answer:
Acrylonitrile monomer
Question 19:
Write the names of allotropes of carbon.
Answer:
Diamond, graphite and fullerene.
Question 20:
Write IUPAC name of (RBSESolutions.com) isobutane.
Answer:
Methylpropane
Question 21:
What is the full form of PAN?
Answer:
Poly Acrylo Nitrile
Question 22:
PVC is made by polymerization of which molecule?
Answer:
Vinyl Chloride
Carbon and its Compounds Short Answer Type Questions
Question 23:
Write any three differences in properties (RBSESolutions.com) of diamond and graphite.
Answer:
| Diamond | Graphite |
|
|
![]()
Question 24:
What do you understand by catenation of carbon atom?
Answer:
Carbon atom has the ability to make large molecules by combining with carbon and many other atoms. It can make long chains. This ability of carbon atom is called catenation.
Question 25:
Write IUPAC name and structural formula of following:
(a) C5H12
(b) C4H8
(c) C3H4
Answer:
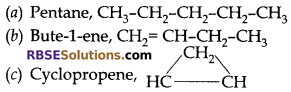
Question 26:
Write any two uses of Freon.
Answer:
Two uses of Freon are
(i) As inert solvent.
(ii) As coolant in refrigerator and cold storage.
Question 27:
Why CNG is better than LPG as (RBSESolutions.com) a fuel?
Answer:
CNG is lighter than LPG. Hence, in case of leakage CNG goes up in air while LPG spreads on ground. Also CNG has less amount of carbon thus, produce negligible amount of carbon monoxide and carbon dioxide on combustion. Due to this CNG is much better than LPG.
Question 28:
Diamond is hard but graphite is soft. Why?
Answer:
Any carbon atom in diamond is bound to four other carbon atoms results in the strong tetrahedral arrangement. In graphite, a carbon atom is bound to three other carbon atoms. It form hexagonal sheets which are comparatively weakly bonded to each other and thus, they can slip pass to each other. Due to this, diamond is hard but graphite is soft.
Question 29:
Write any four (RBSESolutions.com) characteristics of fullerene.
Answer:
Four features of fullerene:
- It appears like a football.
- A molecule of fullerene may contain 60, 70 or more atoms of carbon.
- C60(a fullerene) is bad conductor of electricity.
- It acts as superconductor at high temperature.
Question 30:
Classify hydrocarbons.
Answer:
Hydrocarbons can be classified as acyclic and cyclic hydrocarbons.
(I) Acyclic Hydrocarbons: The carbon chain in these compounds is either linear or branched. These are of two types:
- (a) Saturated Hydrocarbons: Only single bonds are present in these compounds. They are also known as alkanes. General formula of alkane is CnH2n+2, e.g. methane, ethane, butane, etc.
- (b) Unsaturated Hydrocarbons: A double bond or a triple bond is present in these compounds apart from single bonds. Unsaturated hydrocarbons with double bond are called alkenes, e.g. ethene, butene, etc. Unsaturated hydrocarbons with triple bond are called alkynes, e.g. propyne, butyne, etc.
(II) Cyclic Hydrocarbons: The carbon chain in these compounds is in cyclic form. These are of two types:
- (a) Alicyclic Compounds: These compounds bum without smoke, e.g. cyclohexane.
- (b) Aromatic Compounds: These compounds bum with smoke, e.g. benzene.
Question 31:
What are the uses (RBSESolutions.com) of graphite?
Answer:
Following are the uses of graphite:
- Used in making pencil.
- Used as dry lubricant.
- For making electrodes.
- For polishing iron articles.
- As moderator in nuclear furnace.
![]()
Question 32:
Write main characteristics of carbon atom.
Answer:
Following are the main characteristics of carbon atom:
- Valency of carbon atom is 4.
- Carbon can make single bond, double bond and triple bond with other atoms.
- It can make linear, branched chains and cyclic compounds by bonding with other carbon atoms and other elements.
Question 33:
Write IUPAC names (RBSESolutions.com) of following:
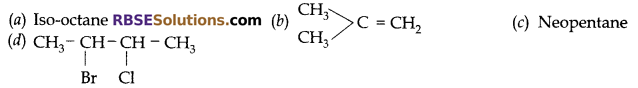
Answer:
(a) 2,2,4-trimethyl pentane
(b) 2-Methylpropene
(c) 2,2-dimethyl propane
(d) 2-bromo-3-chloro butane
Question 34:
What are plastics? Write the names of polymers of main plastics.
Answer:
Plastic is polymer of simple organic molecules that can be moulded into solid objects. PVC, polythene, polystyrene, etc. are examples of plastic.
Question 35:
What is the utility of diamond (RBSESolutions.com) and graphite?
Answer:
Uses of diamond: It is used for making jewellery, for making cutting instruments for glass and rocks.
Uses of graphite: It is used for making pencil, as lubricant, etc.
Question 36:
Explain the nomenclature of Freons.
Answer:
Nomenclature of freon is on the basis of number of carbon, hydrogen and fluorine atoms in molecular formula.
Example: Freon-xyz
Here,
x = number of carbon atoms-1,
y = number of hydrogen atoms + 1
and z = number of fluorine atoms.
Carbon and its Compounds Long Answer Type Questions
Question 37:
What are synthesized polymers? Comment (RBSESolutions.com) on their production and uses.
Answer:
Polymers which are made in laboratory or factory are called synthesized polymers. Some examples are given below.
Nylon-66: This is made after condensation of adipic acid (6-carbon) and hexa methylene diamine (6-carbon) and hence is called nylon-66. It is used for making gear and bearing in machines, tyre, cloth, fibre, ropes, toothbrush, etc.
Terylene: This obtained by condensation of ethylene glycol and terephthalic acid. It is also called decron. It is used for making cloth, sail, belt, magnetic tape, film, etc.
Rayon: For making rayon, paper (cellulose) is dipped in sodium hydroxide solution and cleaned. After that, it is dissolved in carbon disulphide. This solution is passed through fine pores and collected in dilute sulphuric acid. The process gives fine shiny fibres of rayon. It is used for making cloth, threads, carpets, etc.
Polythene: Ethylene is subjected to polymerization under high temperature in presence of catalyst to obtain polythene. This is flexible and strong plastic. It is used for making bags, bottles, pipe, tube, etc.
Poly vinyl chloride: It is obtained by polymerisation of vinyl chloride. It is used for making pipe, shoes, slippers, bags, raincoat, toys, phonogram record, insulating layer, etc.
Poly acrylo nitrile: This is obtained by polymerization of vinyl cyanide. It is used for making sweater, wool-like fibre, pillow, quilt, etc.
Polystyrene: This is obtained by polymerization of vinyl benzene (styrene). It is used for making cups, bottle caps, refrigerator parts, wall tiles, packing materials, etc.
Question 38:
Write short notes on following:
(a) Freon
(b) CNG
(c) Natural rubber
Answer:
(a) When chlorine and fluorine make (RBSESolutions.com) compounds with carbon, the compounds are called chlorofluorocarbons. Poly-chloro-fluoro alkane is called Freon.
Production of Freon: When carbon tetrachloride reacts with hydrogen fluoride in presence of antimony pentachloride, we get Freon-11.
![]()
(b) When natural gas is compressed it is called Compressed Natural Gas (CNG). The gas present above oil layer in oil wells is called natural gas. CNG has less amount of carbon and hence is produces negligible amount of carbon monoxide or carbon dioxide on combustion. So, CNG is more environment-friendly than other petroleum products.
(c) Natural Rubber: This is obtained from latex which is a sap of a tree. Natural rubber is a polymer of isopropene.
Acetic acid is added to latex to make it solid. Rubber obtained by this process is highly elastic and has less tensile strength. This is not suitable for making useful products. To make it useful, rubber is heated with sulphur and the process is called vulcanization. Rubber obtained after vulcanization is strong, inelastic and more durable.
![]()
Question 39:
Answer the following:
(a) Write the rules of (RBSESolutions.com) nomenclature of alkane.
Answer:
Following are the rules of nomenclature of alkane:.
- The longest chain is selected. Group out of the main chain is called substituent.
- When two or more chains of same length are present then the chain with greater number of substituents is selected.
- Name of substituent is first to be written and prefixes are written in alphabetical order.
- If more than one but same substituent are present then mono, di, tri, tetra, penta, etc. are written as per number of substituents.
- Two numbers are separated by comma and hyphen is used between a number and a name.
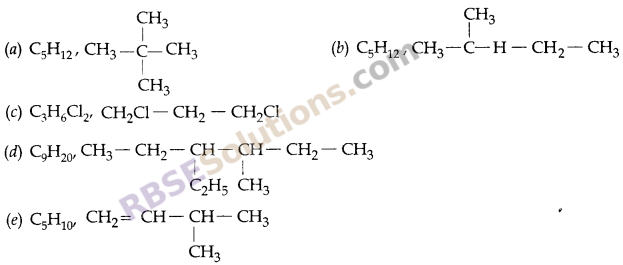
(b) Write the formulae of following:
(1) Neopentane
(2) Isopentane
(3) 1,3-dichloropropane
(4) 3-ethyl-4-methyl hexane
(5) 3-methyl-l-butene
Answer:
Carbon and its Compounds Additional Questions Solved
I. Multiple Choice Questions:
Question 1:
The isomeric (RBSESolutions.com) pair is
(a) ethane and propane
(b) propane and butane
(c) ethane and butane
(d) butane and 2-methyl propane
Answer:
(d) butane and 2-methyl propane
Question 2:
The structural formula of ethyl ethanoate is
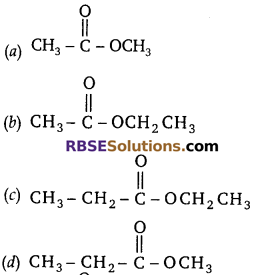
Answer:
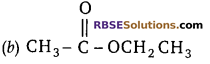
![]()
Question 3:
Identify the product formed when methane reacts with (RBSESolutions.com) chlorine in the presence of sunlight is
(a) C2Cl6
(b) CH3Cl
(c) CHCl4
(d) None of these
Answer:
(b) CH3Cl
Question 4:
Which is denatured spirit?
(a) ethanol only
(b) ethanol and methanol (50%)
(c) ethanol and methanol (5%)
(d) methanol only
Answer:
(c) ethanol and methanol (5%)
Question 5:
According to IUPAC system, the correct name of (RBSESolutions.com) the organic compound is
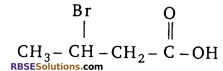
(a) 2-bromobutanoic acid
(b) 2-bromobutysis acid
(c) 3-bromobutanoic acid
(d) 3-bromo-2-hydroxybutan-2-one
Answer:
(c) 3-bromobutanoic acid
Question 6:
Covalent compounds
(a) have high melting and boiling points
(b) are mostly soluble in water
(c) are formed between atoms of metals and non-metals
(d) are formed by the sharing of electrons in the bonding atoms.
Answer:
(d) are formed by the sharing of electrons in the bonding atoms.
Question 7:
Vinegar is a solution of
(a) 30% – 40% acetic acid (RBSESolutions.com) in alcohol
(b) 5% – 8% acetic acid in alcohol
(c) 5% – 8% acetic acid in water
(d) 15% – 20% acetic acid in water
Answer:
(c) 5% – 8% acetic acid in water
Question 8:
The correct electron dot structure of a water molecule is

Answer:
![]()
Question 9:
Bromine reacts with saturated hydrocarbon (RBSESolutions.com) at room temperature in the
(a) absence of sunlight
(b) presence of water
(c) presence of sunlight
(d) presence of hydrochloric acid
Answer:
(c) presence of sunlight
![]()
Question 10:
The number of single and double bonds present in benzene are
(a) 9 and 6
(b) 9 and 3
(c) 12 and 3
(d) 12 and 6
Answer:
(b) 9 and 3
Question 11:
Identify the correct way of numbering an organic (RBSESolutions.com) compound (according to IUPAC).
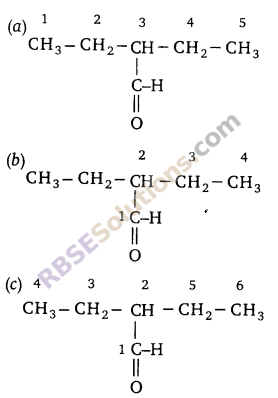
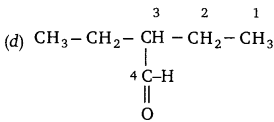
Answer:
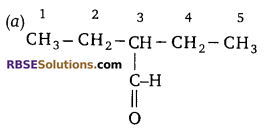
Question 12:
Identify the functional group present in the (RBSESolutions.com) following compound
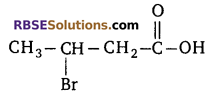
(a) aldehyde
(b) bromine
(c) carboxylic group
(d) both bromine and carboxylic group
Answer:
(d) both bromine and carboxylic group
Question 13:
The upper and lower homologue of C2H5OH are respectively
(a) methyl alcohol and butyl alcohol
(b) ethyl alcohol and propyl alcohol
(c) butyl alcohol and propyl alcohol
(d) propyl alcohol and methyl alcohol
Answer:
(d) propyl alcohol and methyl alcohol
Question 14:
Ethanoic acid was added to sodium carbonate solution (RBSESolutions.com) and the gas evolved was tested with a burning splinter. The following four observations were reported. Identify the correct observation.
(a) The gas bums with pop sound and the flame gets extinguished
(b) The gas does not bum but the splinter bums with pop sound
(c) The flame extinguishes and the gas does not bum.
(d) The gas bums with a blue flame and the splinter bums brightly
Answer:
(c) The flame extinguishes and the gas does not bum.
Question 15:
Which of the following is not a straight chain?
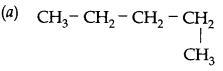
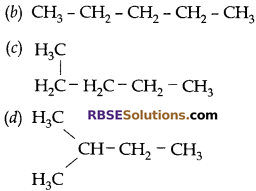
Answer:
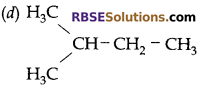
Question 16:
The general formula (RBSESolutions.com) for aldehydes is CnH2n+1CHO. The value of ‘n for the first member.
(a) 1
(b) 0
(c) 0.5
(d) 1.1
Answer:
(b) 0
![]()
Question 17:
An organic compound ‘X’ has the molecular formula C3H6O2. It has a pleasant smell but does not turn blue limus red. It has structural formula
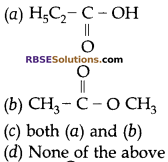
Answer:
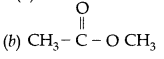
Carbon and its Compounds Very Short Answer Type Questions
Question 1:
What is a hydrocarbon?
Answer:
It is a compound of hydrogen and carbon..
Question 2:
Give different forms in which (RBSESolutions.com) carbon occurs in nature.
Answer:
Carbon occurs in free form as graphite and diamond. In combined form it exists as carbon dioxide, carbonates, etc.
Question 3:
Name two types of hydrocarbon.
Answer:
Hydrocarbon – Saturated and unsaturated.
Question 4:
What are covalent bonds?
Answer:
Bonds which are formed by sharing of a pair of electrons between two atoms is called covalent bonds.
Question 5:
What is catenation?
Answer:
Carbon has the unique ability to form bonds with the (RBSESolutions.com) other atoms of carbon which gives rise to large molecules. This property of self linking is called catenation.
![]()
Question 6:
Name two forms of allotropes of carbon.
Answer:
Two forms of allotropes of carbon are – Crystalline and amorphous
Crystalline form – Diamond and graphite. Amorphous form – Charcoal, coal, coke.
Question 7:
Why covalent compounds have low melting and boiling points?
Answer:
As the bond is formed by sharing of electrons between two atoms. Intermolecular forces are small between the covalent compounds. These bonds break easily.
Question 8:
Identify the following (RBSESolutions.com) compound.
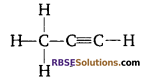
Answer:
Propyne.
Question 9:
Name the following compound.
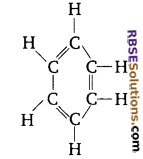
Answer:
Benzene, C6H6.
Question 10:
Give the formula for the (RBSESolutions.com) functional group of aldehyde.
Answer:
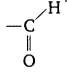
Question 11:
What are heteroatoms?
Answer:
An element or group of elements which replaces one or more hydrogen (H) atoms from hydrocarbon, such that valency of carbon remains satisfied.
Example: CH4 → CH3 – OH
Hence, – OH is a heteroatom.
Question 12:
Name the given compound
Answer:
2 – Butanone.
Question 13:
How can you convert (RBSESolutions.com) ethene into ethane?
Answer:
By adding hydrogen to ethene in the presence of a catalyst.
Question 14:
Give two uses of methane gas.
Answer:
(i) It is used as a fuel
(ii) It is the major component of biogas and CNG.
![]()
Question 15:
What is isomerism?
Answer:
A property in which a compound can exist in different structural formula but its molecular formula remains the same.
Question 16:
Name the second member (RBSESolutions.com) of alkyne series.
Answer:
Propyne
Question 17:
Give the names of the functional group
(i) – CHO
(ii) > C = 0
Answer:
(i) – CHO → Aldehyde
(ii) > C = O
Question 18:
Give the IUPAC name of acetic acid and propyl alcohol.
Answer:
Acetic acid – Ethanoic acid
Propyl alcohol – Propanol
Question 19:
What will happen to the litmus (RBSESolutions.com) solution in carboxylic acid?
Answer:
Red litmus remains the same but blue litmus changes to red.
Question 20:
Give the electron dot structure of CH3Cl and C2H2.
Answer:
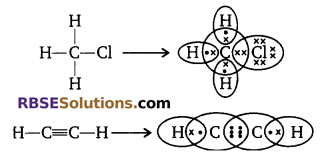
Question 21:
Draw the electron dot structure of N2 and NH3.
Answer:
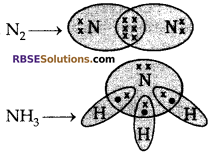
Question 22:
What happens when ethanol (RBSESolutions.com) bums in air?
Answer:
Ethanol bums to form carbon dioxide and water.
Question 23:
Give the IUPAC name and write the functional group present in vinegar.
Answer:
Vinegar IUPAC name is acetic acid, CH3COOH
Functional group – COOH
Question 24:
A compound has a molecular formula C2H6O. It is used as a fuel. Name the compound and name its functional group.
Answer:
C2H6O is an alcohol, i.e. ethanol C2H5OH Functional group is – OH
![]()
Carbon and its Compounds Short Answer Type Questions
Question 1:
What is the reactive site in the given (RBSESolutions.com) hydrocarbon? Write its IUPAC name.
H3C-CH2-CH = CH-CH3
Answer:
The reactive site is at a place where double bond is present.
Name of the compound is 2-pentene.
Question 2:
What is the difference in the number of carbon and hydrogen atoms between two successive members of a homologous series? Also give the difference in their atomic masses.
Answer:
The difference is of 1 carbon and two hydrogen atoms i.e., – CH2 and mass difference is 14 a.m.u.
Question 3:
Why does carbon form compounds mainly by covalent bonding?
Answer:
Carbon forms compounds mainly by covalent bonding because carbon has small size, so neither it can loose four electrons easily, because very high amount of energy will be required, nor it can gain four electrons. Hence, it shares four electrons forming covalent bonds.
Question 4:
Why does carbon forms large number (RBSESolutions.com) of compounds?
Answer:
Carbon forms large number of compounds because of tetravalency and catenation property.
Tetravalency – Carbon has valency 4, to attain noble gas configuration carbon share
its valence electrons with other elements like hydrogen, chlorine, etc.
Catenation – Carbon also shows the property of self-linking in which it forms long, branched or cyclic chains to form large number of compounds.
Question 5:
Write the structural formula for bromopentane and ethanoic acid.
Answer:
Bromopentane (C5H11Br)
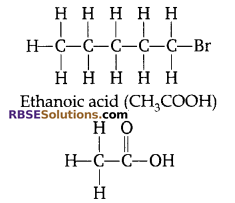
Question 6:
Draw the structures of two (RBSESolutions.com) isomers of butane.
Answer:
Butane C4H10
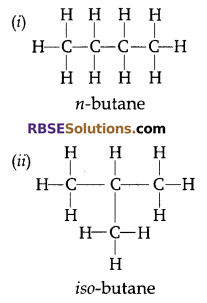
![]()
Question 7:
A student burns a hydrocarbon in air and (RBSESolutions.com) obtains sooty flame. Give two reasons for this observation.
Answer:
Sooty flame could be obtained due to
- Incomplete combustion of saturated hydrocarbons.
- Combustion of unsaturated hydrocarbon.
Question 8:
Differentiate between saturated and unsaturated hydrocarbons. Give one example for each.
Answer:
| Saturated hydrocarbon | Unsaturated hydrocarbon |
| 1. It consists of single bond between carbon atoms. | Double or triple bond between carbon atoms is present. |
| 2. It bums with blue flame. | It burns with sooty flame. |
| 3. Show substitution reaction | Show addition reaction. |
| 4. Less reactive Eg. CH4, Methane C2H6, Ethane | More reactive E.g. H2C=CH2, Ethene HC ≡ CH, Ethyne |
Question 9:
Write the general formula for each of the following (RBSESolutions.com) hydrocarbons and give one example for each.
(i) Alkene
(ii) Alkyne
Answer:
(i) Alkene, CnH2n e.g., C2H4, ethene
(ii) Alkyne, CnH2n-2 e.g., C2H2, ethyne
Question 10:
Diamond and graphite show different physical properties although they are made up of carbon and shows same chemical properties. What is this property called?
Answer:
This property is allotropy.
The physical properties are different because the carbon-carbon bonding in both the cases varies. In diamond, one carbon atom is bonded with four other carbon atoms with strong covalent bond so it is hard, while in case of graphite each carbon forms three strong bonds with other three carbon atoms to form sheet of hexagonal rings and one weak bond is formed between these rings which can slide over each other, so it is soft.
Answer:
X is ethanol, (C2H5OH)
Y is ethanoic acid (CH3COOH)
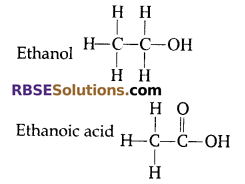
Question 11:
(a) Why are covalent compounds generally (RBSESolutions.com) poor conductors of electricity?
(b) Name the following compound:
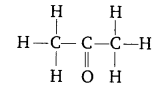
(c) Name the gases evolved when ethanoic ‘ acid is added to sodium carbonate.
How would you prove the presence of this gas?
Answer:
(a) Covalent compound do not form ions. Thus, no carrier of charge is present.
(b) Propanone
(c) Ethanoic acid reacts with sodium carbonate to produce carbon dioxide gas. To prove the presence of this gas allow it to pass through lime water (freshly prepared). It turns lime water milky.

![]()
Carbon and its Compounds Long Answer Type Questions
Question 1:
(a) What do you mean by (RBSESolutions.com) allotropy?
(b) What is isomerism?
(c) Give one example of homologus series, give two properties of it.
(d) What is the full form of IUPAC?
Answer:
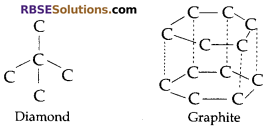
(a) Allotropy: It is the property of an element in which it exists in different forms which show same chemical properties but different physical properties, due to difference in the bonding of atoms
Example: Diamond and graphite are having same chemical properties but they look/appear to be physically different as the bonding in both differs.
(b) Isomerism: It is the property of hydrocarbons (RBSESolutions.com) which show same molecular formula but exhibits different structural formula.
Example: Butane
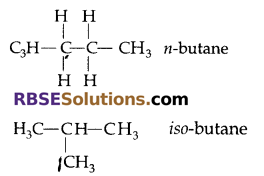
Both of them show different properties,
(c) Homologous series: When the members of a hydrocarbon (RBSESolutions.com) family obey same general formula they are said to be in homologous series. The members in the series are arranged in increasing order of their molecular masses:
Example:
Alkane – CnH2n+2
CH4 – Methane
C2H6 – Ethane
C3H8 – Propane
C4H10 – Butane
Properties:
- The difference between two consecutive members of homologous series is of – CH2 and mass 14 a.m.u.
- They all show same chemical properties and slight gradation in their physical properties.
(d) IUPAC: International Union of Pure (RBSESolutions.com) and Applied Chemistry.
Question 2:
(a) What are hydrocarbons?
(b) Give difference between saturated and unsaturated hydrocarbons.
(c) Why does carbon form large number of compounds?
Answer:
(a) Hydrocarbons – A compound of carbon and hydrogen..
(b)
| Saturated | Unsaturated |
|
C = C, C ≡ C double or triple bond. Alkenes CnH2n, and Alkynes CnH2n+2 Undergo addition reaction Burns with sooty flame |
(c) Carbon forms large number of (RBSESolutions.com) compounds due to
- Catenation – Self linking property which leads to long straight chains, branched chains and cyclic chains.
- Isomerism – Compound of carbon can exist in more than one structural formula but has same molecular formula.
- Tetravalency – To acquire noble gas configuration, carbon shares its outer electrons with other elements, thus form covalent bond with other elements.
Question 3:
Give a brief overview of different systems of nomenclature of organic compounds.
Answer:
There are three methods of nomenclature of organic compounds:
Trivial System: In this system, nomenclature of a compound is based on natural source and properties of compound. Some examples are:
CH3OH – wood spirit
CH3COOH – Marsh gas
Derived System: In this system, organic compounds (RBSESolutions.com) are named as derivatives of their simpler compounds. Some examples are.
CH3CH2OH – Methyl carbinol
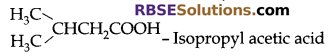
IUPAC System: Following rules are obeyed while naming a compound:
- The prefix of compound is based on number of carbon atoms in a molecule of given compound.
- The suffix is written on the basis of type of bond in the compound.
- Name of compound is written after using appropriate prefix or suffix.
- Prefixes meth, eth, prop, but, pent, etc. are used respectively for one, two, three, four or five carbon atoms in the chain. Suffixes ane, ene and yne are respectively used for single, double and triple bonds.
![]()
Question 4:
Write a short note on CNG and differentiate (RBSESolutions.com) between CNG and LPG.
Answer:
When natural gas is compressed it is called Compressed Natural Gas (CNG). The gas present above oil layer in oil wells is called natural gas. CNG has less amount of carbon and hence is produces negligible amount of cabron monoxide or carbon dioxide on combustion. So, CNG is more environment-friendly than other petroleum products.
| LPG | CNG |
| Liquefied petroleum gas is obtained after fractional distillation of crude oil. | CNG is obtained from natural gas from oil well. |
| LPG is heavier. | CNG is lighter. |
| LPG is less safe; it spreads near ground on leakage. | CNG is much safer; it spreads in air on leakage. |
| LPG is mainly used as domestic fuel. | CNG is mainly used as fuel for automobiles. |
Question 5:
What is nylon-66? Give the reaction of production of nylon. (RBSESolutions.com) Write some uses of nylon.
Answer:
Nylon-66: This is made after condensation of adipic acid (6-carbon) and hexa methylene diamine (6-carbon) and hence is called nylon-66.
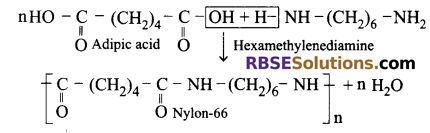
Uses of Nylon:
- For making gear and bearing in machines.
- For making tyre, cloth, fibre, ropes, toothbrush, etc.
Question 6:
Write short notes on (RBSESolutions.com) following:
(a) Polythene
(b) Polyvinyl chloride
(c) Polyacrylo nitrile
(d) Polystyrene
Answer:
(a) Polythene: Ethylene is subjected to polymerization under high temperature in presence of catalyst to obtain polythene. This is flexible and strong plastic. It is used for making bags, bottles, pipes, tubes, etc.

(b) Polyvinyl Chloride: It is obtained by polymerisation of vinyl chloride. It is used for making pipes, shoes, slippers, bags, raincoat, toys, phonogram record, insulating layer, etc.

(c) Poly acrylo nitrile: This is obtained by polymerization of vinyl cyanide. It is used for making sweater, wool-like fibre, pillow, quilt, etc.
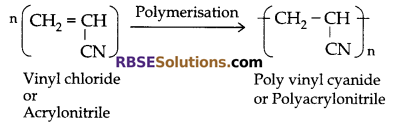
(d) Poly-styrene: This is obtained by polymerization of vinyl benzene (styrene). It is used for making cups, bottle caps, refrigerator parts, wall tiles, packing materials, etc.

We hope the given RBSE Solutions for Class 10 Science Chapter 8 Carbon and its Compounds will help you. If you have any query regarding Rajasthan Board RBSE Class 10 Science Solutions Chapter 8 Carbon and its Compounds, drop a comment below and we will get back to you at the earliest.