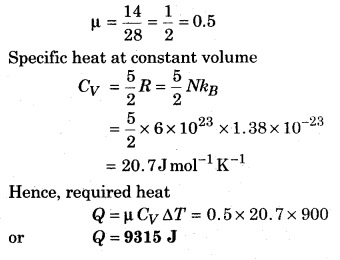Rajasthan Board RBSE Class 11 Physics Chapter 14 Kinetic Theory of Gases
RBSE Class 11 Physics Chapter 14 Textbook Exercises With Solutions
RBSE Class 11 Physics Chapter 14 Very Short Answer Type Questions
Question 1.
What is the value of root mean square speed at a temperature T for an ideal gas?
Answer:
Root mean square speed at a temperature ‘T’ for an ideal gas.
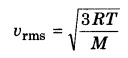
where M = molar mass of the gas; T = absolute temperature of the gas; R = universal gas constant.
Question 2.
What is the unit of gas constant (R)?
Answer:
Ideal gas equation
PV = nRT
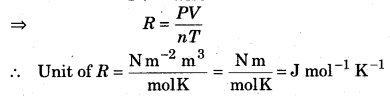
Question 3.
How much would the root mean square speed become when the absolute temperature of a gas is increased by 16 times?
Answer:
Root mean square speed
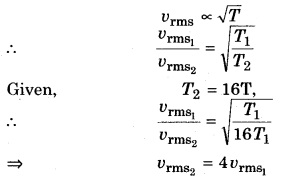
Question 4.
Write the Vander Waals equation.
Answer:
van der Waals equation
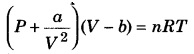
where P pressure, V = volume
T = absolute temperature
a&b = van der Waals constant
Question 5.
According to the kinetic theory of gas, what is the speed of gas molecules at absolute zero temperature?
Answer:
According to the kinetic theory of gas, the root mean square speed of gas molecules is
![]()
At absolute zero temperature i.e.,T = 0
υ2rms = 0 or υrms = 0
Question 6.
Give the relation between degree of freedom (f) and γ.
Answer:
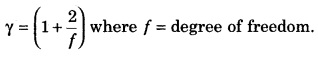
Question 7.
What is the degree of freedom for an airplane flying in the sky?
Answer:
An airplane can have independent velocities in three directions (X,Y,Z). Hence, the degree of freedom is 3.
Question 8.
Give the value of Cp for a diatomic gas.
Answer:
For a diatomic gas,
CP = \(\frac { 7 }{ 2 } \) R, where R = Universal gas constant
Question 9.
Write the relation for pressure by kinetic theory of gases.
Answer:
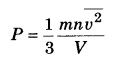
where m is_molar mass; n is number density of molecules; υ2 mean square speed; V = volume of the gas; ρ = density of the gas.
Question 10.
What is the value of Avogadro’s number?
Answer:
NA = 6.023 × 1023.
RBSE Class 11 Physics Chapter 14 Short Answer Type Questions
Questions 1.
Explain briefly the postulates of kinetic theory of gases.
Answer:
The main assumptions of kinetic theory of gases are :
- A gas is made up of a large number of sub-microscopic particles called atoms.
- Gases consist of particles in constant random motion. They continue to move in a straight line until they collide with each other on the walls of their container.
- Gas pressure is due to the moleiMIeS colliding with the walls of the container. All of these collisions are perfectly elastic. This means that there is no change in energy of either the particles or the wall upon collision. So, no energy is lost or gained from collisions.
- No molecular forces are at work. The potential energy of molecules is zero. So, whole of the energy. in an ideal gas is kinetic energy only. There is no attraction or repulsion between the particles.
- The time taken for the collision is negligible as compared with the time between collisions.
- The kinetic energy of a gas is a measure of its kelvin temperature. Each gas molecule has different speed but the temperature and kinetic energy of gas refer to the average of these speeds.
- The average kinetic energy of an atom of gas is directly proportional to the temperature. An increase in temperature increases the speed in which the gas molecules move.
- All gases at a given temperature have same average kinetic energy.
- fighter molecules of a gas move faster than the heavier molecules.
Question 2.
Discuss the physical properties of a gas according to the postulates of kinetic theory of gases.
Answer:
The physical properties of gases on the basis of kinetic theory can be explained as :
1. According to the kinetic theory of gases, the gas molecules have free space between them as they are far away from each other. So, gases can be easily compressed, and if two gas containers are joined, then the density in both the gas containers will become equal.
2. As there is no force of attraction between the gas molecules and there is more kinetic energy due to which gas molecules move to the free space available. That is why, gases have no fixed shape and no fixed volume. Thus, gases get completely filled in the container in which they are kept.
Example: When the lid of a perfume bottle is open, the aroma gets filled in the room.
Question 3.
Describe the temperature according to the kinetic theory of gases.
Answer:
Let the volume of a gas of a moleculfes of 1 g at absolute temperature T is V. Then,
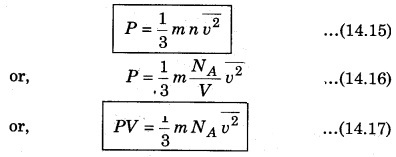
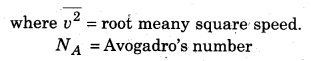
Question 4.
Describe the vander Waals gas equation for real gases.
Answer:
The ideal gas law is based on the assumption that the gases are made up of point masses that undergo perfectly elastic collisions. However, real gases deviates from those assumptions at low temperatures or high pressures. Consider a container where the pressure is increased. The volume of the container decreases, when the pressure is increased. The volume occupied by the gas particles is no longer negligible compared to the volume of the container and the volume of the gas particles need to be considered.
At low temperatures, the gas particles have lower kinetic energy and do not move fast. The gas particles are affected by the intermolecular forces that act on them,which leads to inelastic collisions between them. This, leads to lesser collisions with the container and a lower pressure than that is expected from the ideal gas.
The van der Waal’s equation modifies the ideal gas law. This law predicts the properties of real gases by describing particles of non-zero volume governed by pairwise attractive forces.
The van der Waals equation of state is written as :
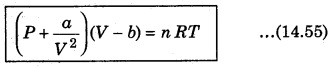
where P is pressure, V is volume, R is universal gas constant and T is absolute temperature.
Fig. 14.4 shows a curve plotted between PV and P in which behaviour of two gases nitrogen and hydrogen has been shown.
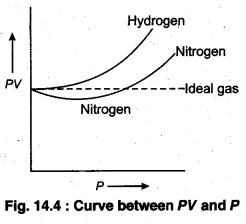
Vander waal’s introduces the necessary corrections in the equation PV = RT.
Question 5.
What do you understand by degree of, freedom?
Answer:
A degree of freedom is an independent physical parameter in the formal description of the state of a physical system.
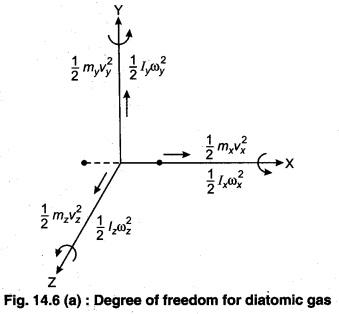
In three-dimensional space, three degrees of freedom are associated with the movement of a particle.
A diatomic gas molecule thus has six degrees of freedom. This set may be split in terms of translations, rotations, and vibrations of the molecule. The centre of mass motion of the whole molecule accounts for three (3) degrees of freedom. Also, the molecule has two rotational degrees of motion and one vibrational degree of freedom.
Question 6.
Explain Boyle’s law from kinetic theory of gases.
Answer:
According to kinetic theory, the average kinetic energy of molecules of a gas is directly proportional to the absolute temperature of gas. So,
![]()
where α = proportionality constant
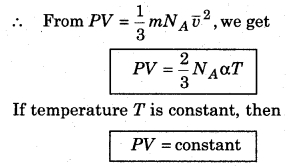
i.e., the product of pressure and volume of gas at constant temperature is constant. This is Boyle’s law.
Question 7.
Explain law of equipartition of energy.
Answer:
The law of equipartition of energy states that, “for any dynamical system in a thermal equilibrium, the total energy is equally divided among the each degrees of freedom.”
The energy related with a molecule for one degree of freedom is \(\frac { 1 }{ 2 } \) KB T where kB is Boltzmann’s constant and T is absolute temperature.
Question 8.
Determine the values of CP, CV and γ for a monoatomic, diatomic and polyatomic gas.
Answer:
For a given mass of a gas, the amount of heat required to produce temperature difference is different for different substances. Let Q be the heat required to increase temperature by ∆T of a gas of mass m, then the specific heat can be defined as follows :
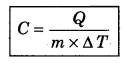
The unit of specific heat in CGS system is cal g-1 C-1 and in MKS unit, it is represented as J kg-1 K-1 For example the specific heat for water is 4.2 × 103 J kg-1 K-1. The specific heat for gas can be from zero to infinity because if we compress a gas without heating it, then it increases the temperature.
For an adiabatic process,
Q = 0 and ∆T = positive, then C = 0
If on heating the gas, the gas expands such that there is no increase in the temperature, i.e., for isothermal process, then, from equation (14.72)
Generally, to determine the amount of specific heat of a gas, pressure or volume is kept constant. Generally, there are two types of specific heats :
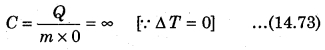
- Specifie Heat at Constant Volume
- Specifie Heat at Constant Pressure
Question 9.
Explain mean free path for a gas molecule.
Answer:
According to the kinetic theory, the molecules of a gas move in different directions and collide with each other.
The mean free path is the average distance travelled by a moving particle (such as an atom, a molecule, a photon) between successive collisions, that modify its direction.
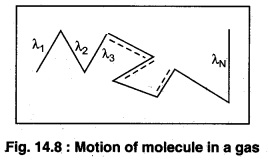
Let the distances covered by molecules of gas in N collisions be λ1, λ2, λ3,…..λN. Then, the mean free path can be defined as
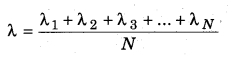
If t is the time taken to cover the path and υ is the mean speed of molecule, then
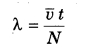
Question 10.
What would be the change in pressure if the number of molecules in a container gets doubled?
Answer:
Pressure of the gas
![]()
When the number of molecules gets doubled i.e., n’ = 2n, the new pressure
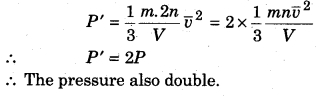
RBSE Class 11 Physics Chapter 14 Long Answer Type Questions
Question 1.
Give the laws of Boyle, Charles, Gay-Lussac and Dalton by writing the postulates of kinetic theory of gases.
Answer:
The main assumptions of kinetic theory of gases are :
1. A gas is made up of a large number of sub-microscopic particles called atoms.
2. Gases consist of particles in constant random motion. They continue to move in a straight line until they collide with each other on the walls of their container.
3. Gas pressure is due to the moleiMIeS colliding with the walls of the container. All of these collisions are perfectly elastic. This means that there is no change in energy of either the particles or the wall upon collision. So, no energy is lost or gained from collisions.
4. No molecular forces are at work. The potential energy of molecules is zero. So, whole of the energy. in an ideal gas is kinetic energy only. There is no attraction or repulsion between the particles.
5. The time taken for the collision is negligible as compared with the time between collisions.
6. The kinetic energy of a gas is a measure of its kelvin temperature. Each gas molecule has different speed but the temperature and kinetic energy of gas refer to the average of these speeds.
7. The average kinetic energy of an atom of gas is directly proportional to the temperature. An increase in temperature increases the speed in which the gas molecules move.
8. All gases at a given temperature have same average kinetic energy.
9. fighter molecules of a gas move faster than the heavier molecules.
According to the kinetic theory, the average kinetic energy of molecules of a gas is directly proportional to the absolute temperature of the gas. So, from equation (14.21),
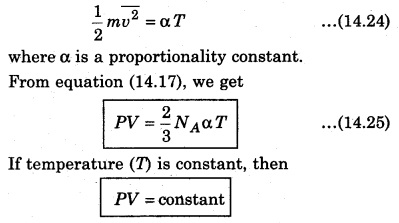
i.e., the product of pressure and volume of gas at constant temperature is constant. This is Boyle’s law.
From equation (14.25), if pressure (P) of a gas is constant, then
V ∝ T …(14.26)
i. e., for a gas of a given mass, volume of gas is directly proportional to the temperature at constant pressure. This is Charles’ law.
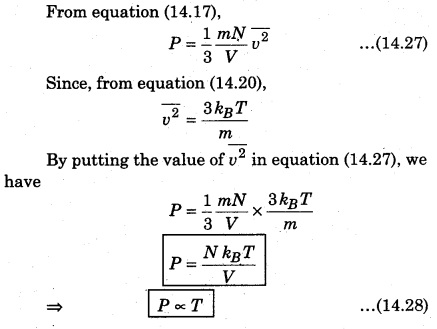
So, at constant volume for a gas of given mass, the pressure of a gas is directly proportional to the temperature of gas. This is Gay Lussac’s law.
According to Dalton’s law of partial pressure, in thermal equilibrium state, the total pressure of each gas is equal to the sum of the different pressures of each gas for a mixture of unreactive gases filled in a container, i.e., if P1,P2,P3,… respectively are the different pressure of gases. Then, the total pressure (P)
P = P1 + P2 + P3 +…. (14,41)
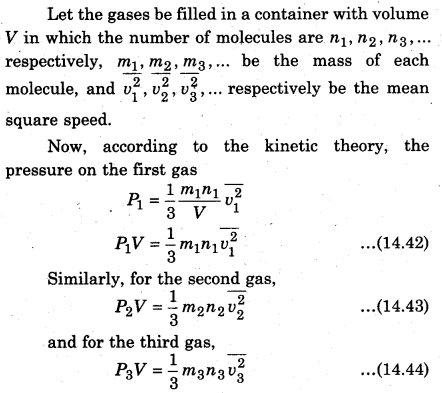
Similarly, we can write equation for other gases. Since the mixture of all- the gases are in thermal equilibrium state, i.e., the temperature is same. Then, average kinetic energy of each molecule of a gas will be equal, i.e.,
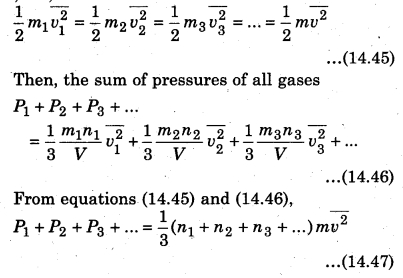
If the total pressure of container is P and the total number of molecules are (n1 + n2 + n3 +…), then the total applied pressure,
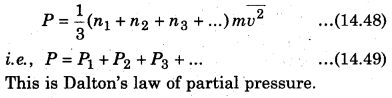
Question 2.
Calculate the pressure on the walls of container filled with gas according to kinetic theory.
Answer:
Consider a gas enclosed in a cube. Let the axes be parallel to the sides of the cube (Fig. 14.3).
A molecule having velocity (Vx ,Vy,Vz) hits the planar wall parallel to γz plane of area A(l2). As the collision is elastic, the molecule rebounds with the same velocity, y and z components of velocity do not change in the collision but the sign of x-component is reversed. Then, the velocity of collision is (-Vx, Vy, Vz).
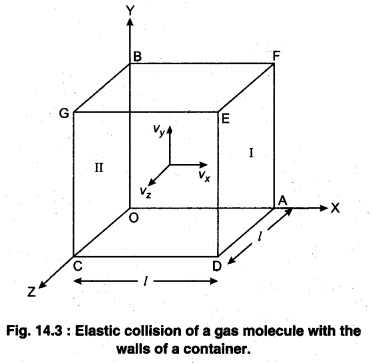
The change in momentum of molecule,
= -mVx – (mVx) = -2 mVx …(14.9)
According to the principle of conservation of momentum. The momentum imported to wall (in collision) = 2mVx. Consider a small time interval ∆ t, a moljecule with velocity Vx will hit the wall when it is within the volume of AVx∆t and can hit the wall in time ∆t. The number of molecules with velocity (Vx, Vy, Vz) hitting the wall in time ∆t is AVx∆ t n where n is the number of molecules per unit volume.
The total momentum transferred to wall by these molecules in time ∆t is :
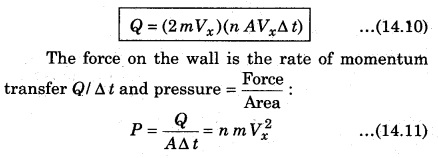
There is a distribution in velocities. So, equation (14.11) stands for pressure due to the group of molecules having velocity Vx in x-direction and n is the number density of these molecules.
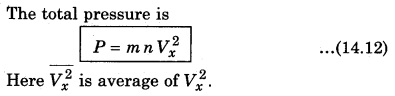
If the gas is isotropic, i.e., the velocity of molecules in the vessel has no preferred direction.
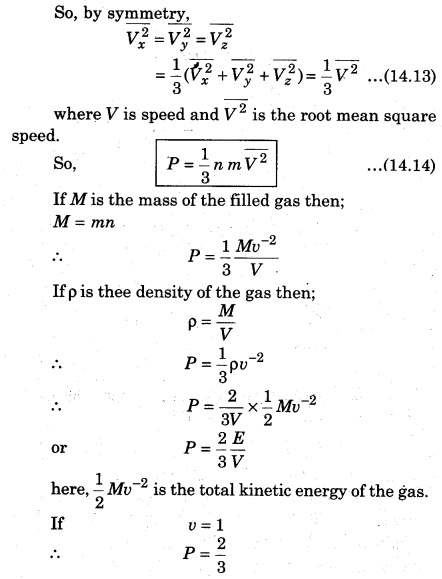
Question 3.
What is meant by degree of freedom. Explain the specific heats for monoatomic, diatomic and polyatomic gases.
Answer:
A degree of freedom is an independent physical parameter in the formal description of the state of a physical system.
In three-dimensional space, three degrees of freedom are associated with the movement of a particle.
A diatomic gas molecule thus has six degrees of freedom. This set may be split in terms of translations, rotations, and vibrations of the molecule. The centre of mass motion of the whole molecule accounts for three (3) degrees of freedom. Also, the molecule has two rotational degrees of motion and one vibrational degree of freedom.
Since in a monoatomic gas, each molecule of the gas have only translatory motion and hence its degree of freedom is 3, i. e., f = 3,
Then from equations (14.78), (14.79) and (14.81)
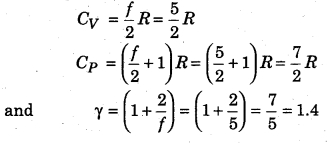
We know that each molecule of a diatomic gas is made up of two molecules, in which degree of freedom of each molecule is 5, i. e., f = 5
From equations (14.78), (14.79) and (14.81),
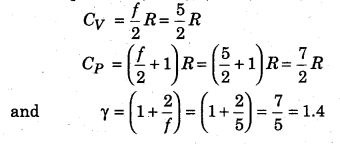
Since a polyatomic has three degrees of freedom each of translation and rotation. Also, there is some definite number of vibrational degrees of freedom (fv). Then, total degree of freedom per molecule (f),
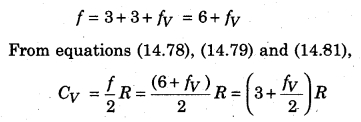
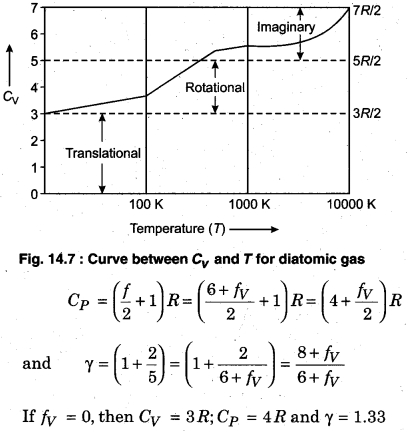
The graph plotted between Cv and T for diatomic gases,is shown in Fig. 14.7. We see that the temperature increases, the gas absorbs heat through the translational, rotational and vibrational ways.
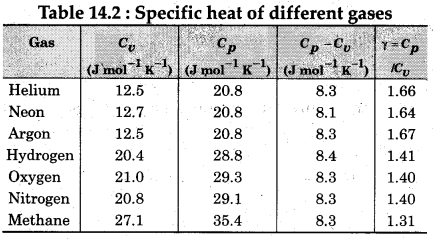
Question 4.
Differentiate between ideal gases and real gases.
Answer:
The three states of matter that are recognised by their characteristics are solids, liquids and gases. Solids have definite mass and shape due to the strong molecular attraction. In liquids the molecules are moving so they result in taking the shape of the container. In gases the molecules are free to move anywhere in the container. Two types of gases exists. Real gas and Ideal gas. As the particle size of ideal gas is extremely small and the mass is almost zero and no volume Ideal gas is also considered as point mass. The molecules of real gas occupy space though they are small particles and also has volume.
Ideal gas:
Ideal gas is defined as a gas that obeys gas laws at all condition of pressure and temperature. Ideal gases have velocity and mass. They do not have volume. When compared to the total volume of the gas the volume occupied by the gas is negligible. It does not condense and does not have triple point.
Real gas:
Ideal gas is defined as a gas that does not obey gas laws at all standard pressure and temperature conditions. When the gas becomes massive and voluminous it deviates from its ideal behaviour. Real gases have velocity, volume and mass. When they are cooled to their boiling point, they liquefy. When compared to the total volume of the gas the volume occupied by the gas is not negligible.
To make you understand how ideal gas and real gas are different from each other, here are the some of the major differences between ideal gas and real gas:
Difference between Ideal gas and Real gas:
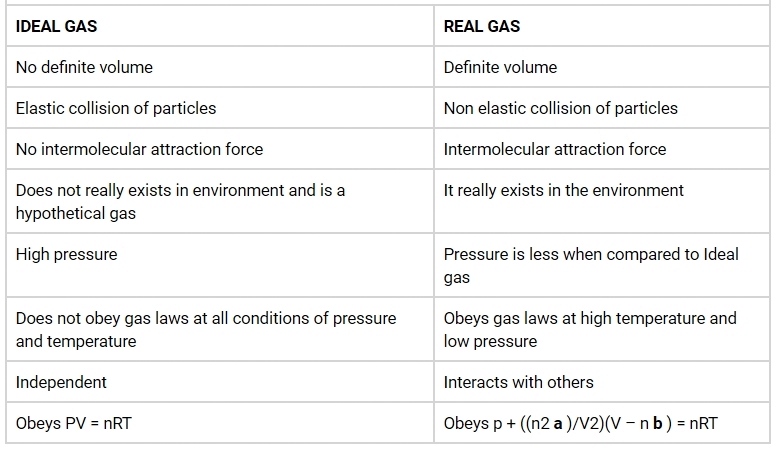
Question 5.
Derive the relation between kinetic energy of ideal gas and temperature.
Answer:
We know that if heat is not transferred when the two systems are in contact, then it is said to be in thermal equilibrium state and their temperature is same.
Let 1g molecule of gas with volume and temperature be V and T respectively and number of molecules of 1 mol of gas (NA) = 6.02 × 1023 molecules. According to kinetic theory of gases, the pressure of gas for 1 g molecules is :
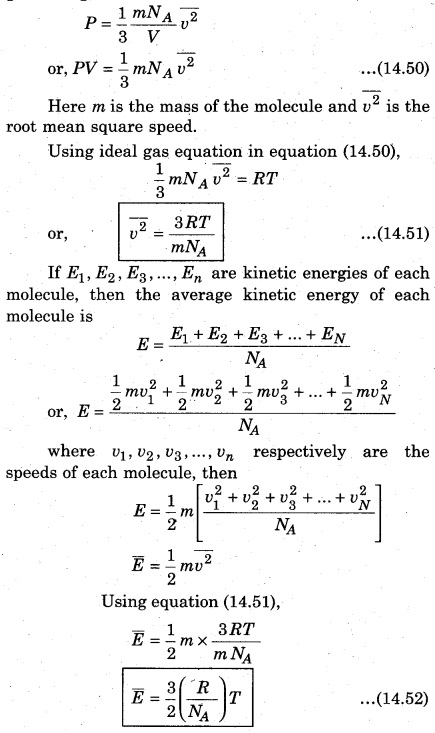
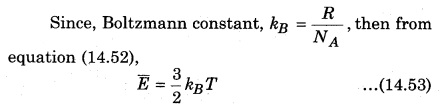
Equation (14.53) is a relation between the average kinetic energy of each molecule and temperature. It is clear that E and T are directly proportional to each other, i. e.,
![]()
i. e., kinetic energy of each molecule of a gas is a measure of temperature and at absolute zero temperature, i.e.,
![]()
So, at absolute zero temperature, the molecules are motionless. Thus the absolute zero temperature is the temperature at which the speed of all molecules is zero.
RBSE Class 11 Physics Chapter 14 Numerical Example
Question 1.
Calculate the root mean square speed of a gas at 300 K if the molar mass of gas is 221 and R = 8.3 J mol-1 K-1.
Solution:
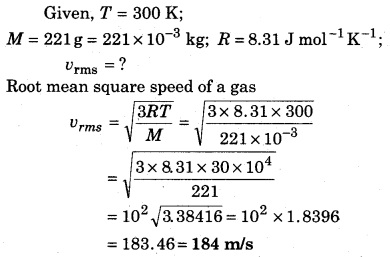
Question 2.
If the density of nitrogen is 1.25 gL-1 at NTP, then calculate the root mean square speed at 0°C and 20°C.
Solution:
Volume of 1 mol of gas at NTP = 22.4 L and temperature T = 273 K,
Given: density of nitrogen,
P = 1.25 g L-1
∴ Molecular mass of nitrogen M = PV
= 1.25gL-1 × 22.4L = 28g
∴ Root mean square speed of gas at 0°C is
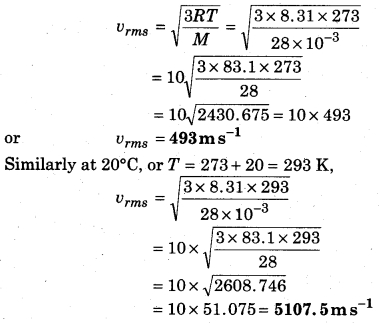
Question 3.
At what temperature, the kinetic energy of a molecule is 1.0 eV? (kg = 1.38 × 10-23 J K-1)
Solution:
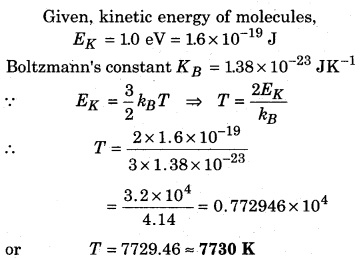
Question 4.
The Vander Waals constant for a gas are a = 1.32 and b = 3.12 × 10-2. Then, determine the temperature at 5 atm pressure and volume 20 L for a 5 mol gas. Also, calculate the gas pressure when the volume is 2 L.
(R = 8.314 J mol-1 K-1)
Sollution:
Given,
P = 5 atm = 5 × 1.013 × 105 N m-2
µ = 5mol; V = 20 L; α = 1.32atm;
b = 3.12 × 10-2 = 0.0312; R = 8.314 J mol-1 K-1; T = ?
From Vander Waals Equation

Question 5.
The Vander Waals constants for oxygen gas are α = 1.32 and b = 3.12 × 10-12. If the temperature of gas is 300 K, its volume is 1.2 L mol-1, then calculate the gas pressure. (R = 8.314 J.mol-1 K-1)
Solution:

Question 6.
A flask is filled with argon and Chlorine gas. Their masses are in the ratio 2 : 1 and the temperature of the mixture is 27°C. Calculate the following for both the gases :
(i) Average kinetic energy per molecule.
(ii) Ratio of root mean square speed of molecules. Here the atomic mass of argon is 39.94 and that of chlorine atom is 70.94.
Solution:
(i) In the mixture, temperature of both the gases is same and the velocity of gas molecules is same
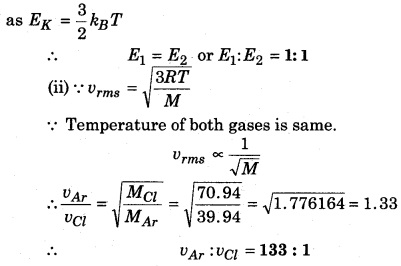
Question 7.
Calculate the mean free path of water molecules in steam at 373 K if the diameter of water molecules is 2 × 10-10 m.
Solution:
Mean free path of molecules
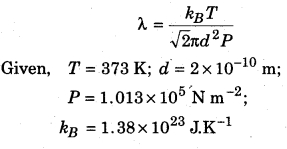
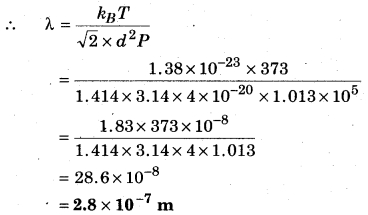
Question 8.
If the temperature of air increases from 127°C to 227°C. In what ratio would the kinetic energy of its molecules increases?
Solution:
T1 = 127°C = 127 + 273 = 400 K;
T2 = 227 + 273 = 500 K
∵ Kinetic energy of molecules
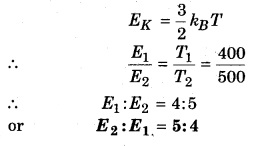
Question 9.
If a monoatomic gas of 1 mol (γ = \(\frac { 5 }{ 3 } \)) is mixed with 1 mol of diatomic gas (γ = \(\frac { 7 }{ 5 } \)), then determine the value of γ for the mixture
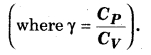
Solution:
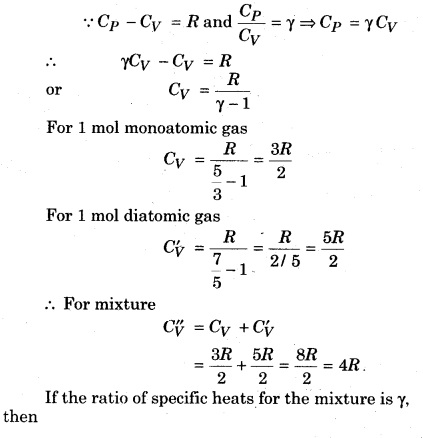
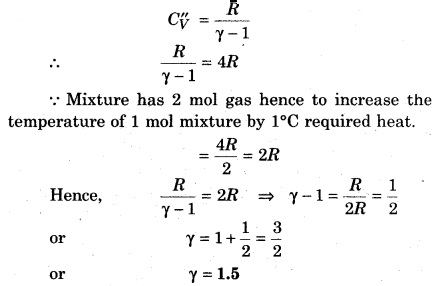
Question 10.
A container has a mixture of 16 g helium and 16 g oxygen. Then, calculate γ for the mixture.
Solution:
For a monoatomic gas
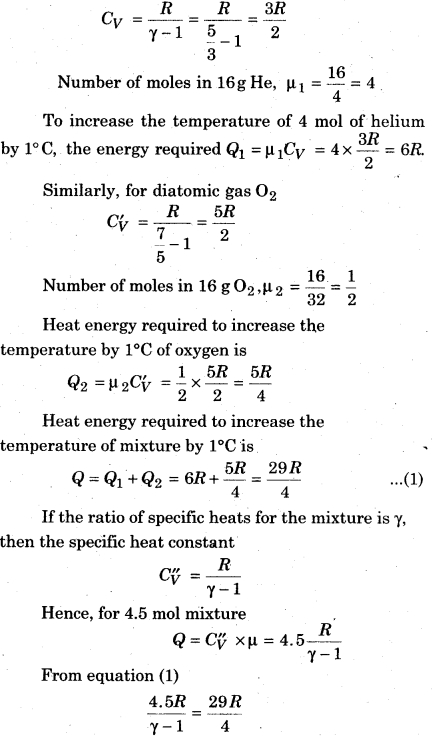
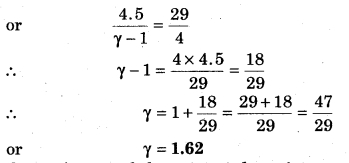
Question 11.
A vessel has 0.014 kg nitrogen gas filled at 27°C. How much heat would be provided to increase the root mean square speed by two times?
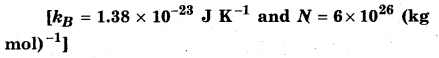
Solution:
Initial temperature of gas
T = 273 + 27 = 300 K
∵ Root mean square velocity, υrms ∝ \(\sqrt { T }\)
Hence, to double the r.m.s. velocity the temperature should be 4 times the intial velocity.
Hence, increase in temperature
= 4T – T = 3T = 3 × 300 = 900 K
Molecular weight of nitrogen is 28, hence mass of 1 mol is 28.
Hence in 0.014 kg or 14 g nitrogen, number of moles are;