Rajasthan Board RBSE Class 12 Chemistry Chapter 10 Halogen Derivatives
RBSE Class 12 Chemistry Chapter 10 Text Book Questions
RBSE Class 12 Chemistry Chapter 10 Multiple Choice Questions
RBSE Solutions For Class 12 Chemistry Chapter 10 Question 1.
Out of the following compounds which compound will give Haloform reaction?
(a) Methanol
(b) Ethanol
(c) 1 – Propanol
(d) Butanol
RBSE Class 12 Chemistry Chapter 10 Question 2.
What happens in Finkelstein reactions?
(a) Dehydrohalogenation
(b) Hydrogenation
(c) Halogen exchange
(d) Oxidation
Halogen Derivatives Class 12 In Hindi Question 3.
Example of haloarene is:
(a) CH3Cl
(b) C6H – CH2Cl
(c) C6H6Cl6
(d) C6H5Cl
Halogen Derivatives Class 12 Question 4.
Which compound will form a yellow precipitate with AgNO3?
(a) CHI3
(b) CH3l
(c) CHCl3
(d) CH3 – CH2I
RBSE Solutions For Class 12 Chemistry Question 5.
The intermediate formed in carbonyl amine reaction is:
(a) CN–
(b) N = C–
(c) CCl2
(d) Cl–
RBSE Solutions 12 Chemistry Question 6.
What forms in SN2 mechanism?
(a) Transition state
(b) Carbonium ion
(c) Carbanion
(d) Free radical
RBSE Solution Class 12 Chemistry Question 7.
Out of the following which compound has zero dipole moment?
(a) CH3Cl
(b) CHCl3
(c) CCl4
(d) CHI3
Answers:
1. (b)
2. (c)
3. (d)
4. (a)
5. (c)
6. (a)
7. (c)
RBSE Class 12 Chemistry Chapter 10 Very Short Answer Type Questions
Halogen Derivatives Class 12 Questions And Answers Question 1.
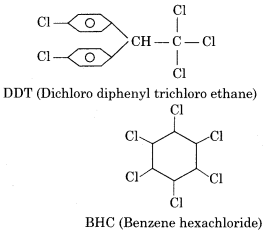
Write full form or name of DDT and BHC.
Answer:
DDT = Dichloro diphenyl trichloroethane
BHC = Benzene hexachloride
Haloalkanes And Haloarenes Questions With Answers Pdf Question 2.
Write name and formula of anyone tertiary alkyl halide.
Answer:
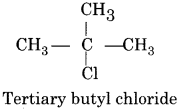
RBSE Solutions Class 12 Chemistry Question 3.
Write the name and formula of anyone alcohol and one ketone which give haloform reaction:
Answer:
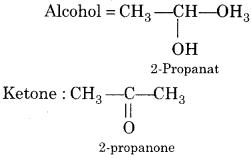
RBSE Solutions Chemistry Class 12 Question 4.
Which reagent is used for the formation of methanol from methyl chloride?
Answer:
Methanol can be prepared by hydrolysis of methyl chloride with aqueous KOH solution.
![]()
Question 5.
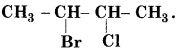
Write the IUPAC name of
Answer:
Chemistry Class 12 RBSE Solutions Question 6.
Give any three examples of electrophiles and nucleophiles?
Answer:
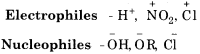
RBSE Solution Class 12th Chemistry Question 7.
Which compound is used in a fire extinguisher?
Answer:
Carbon tetrachloride (CCl4) or tetrachloromethane is used in the fire extinguisher.
Class 12 Chemistry RBSE Solutions Question 8.
Write the formula of DDT and BHC.
Answer:
Chemistry RBSE Solutions Question 9.
Write the possible dichloro derivatives of propane.
Answer:
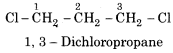
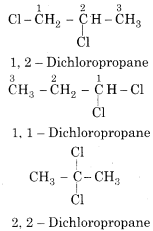
RBSE Solutions For Class 12 Physics Chapter 10 Question 10.
Write Hunsdiecker reaction.
Answer:
Hunsdiecker Reaction – Silver salt of fatty acid can be converted into bromo alkane by refluxing with bromine in the presence of carbon tetrachloride. This reaction is known as Borodine Hunsdiecker reaction or simply Hunsdiecker reaction.
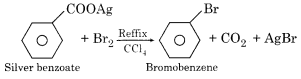
Question 11.
Write formula and uses of chloropicrin and chlorine.
Answer:
(i)
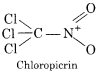 Uses:
Uses:
Chloropicrin was manufactured for use as a poison gas in world war. In agriculture, chloropicrin is injected into the soil prior to planting a crop in order to fumigate soil.
(ii)
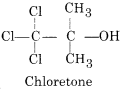 Uses:
Uses:
It acts as a hypnotic and nervous sedative.
Question 12.
Which is the best reagent to obtain pure chloroform?
Answer:
Chloral hydrate is the best reagent to obtain pure chloroform.
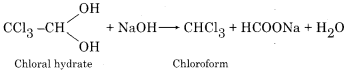
Question 13.
Which gas is formed when chloroform is exposed to air?
Answer:
Poisonous gas phosgene is formed.
![]()
Question 14.
Which one is more reactive from methyl chloride and methyl iodide?
Answer:
CH3 – I will be more reactive than CH3 – Cl because I– ion is a better leaving group than Cl– ion. Alternatively, bond dissociation enthalpy of C – I bond is less than that of the C – Cl bond.
Question 15.
Write the structure of C5H12 which forms only one mono chloro derivative.
Answer:
It must be of symmetrical nature and is neopentane (CH3)4C.
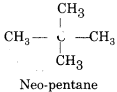
Question 16.
What is the use of DDT?
Answer:
It is used as an insecticide for killing or controlling mosquitoes, lice etc.
Question 17.
Write two examples of 2° alkyl halide.
Answer:
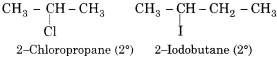
Question 18.
Arrange the following in order of reactivity towards SN1 reaction.
Answer:
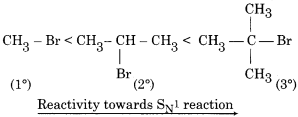
RBSE Class 12 Chemistry Chapter 10 Short Answer Type Questions
Question 1.
C6H5Cl is less reactive towards nucleophilic substitution reactions than C2H5Cl. Explain.
Answer:
In chloroethane, the C-atom of C – Cl bond is sp3 hybridised while in C6H5Cl, the carbon atom is in sp2 hybridisation state. The sp2 hybridised C – an atom with a greater s-character is more electronegative and can hold the electron pair of the C – Cl bond more tightly than sp3 – hybridised C-atom in chloroethane with less s-character. Thus the bond cleavage in chlorobenzene is more difficult and is, therefore, less reactive as compared to chloroethane.
Question 2.
How ethyl bromide is prepared from Grignard reagent?
Answer:
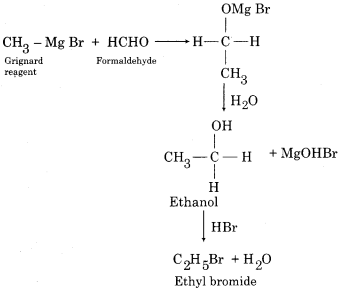
Question 3.
Write the chemical reaction for the formation of BHC.
Answer:
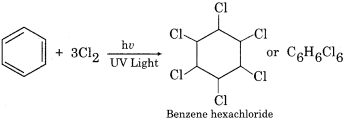
Question 4.
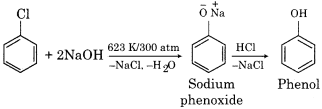
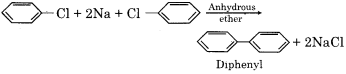
How will you prepare the following compounds from chlorobenzene:
(a) Phenol
(b) Diphenyl
(c) Toluene
Answer:
(a) Phenol from chlorobenzene
(b) Diphenyl from chlorobenzene
(c) Toluene from chlorobenzene
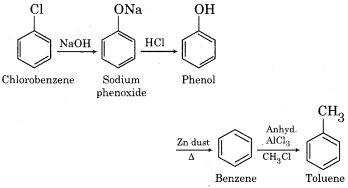
Question 5.
Explain β -elimination.
Answer:
When haloalkane or alkyl halide with a β – hydrogen atom is heated with alcoholic solution of potassium hydroxide (alc. KOH), the OH– ion acts as a base and eliminate an electron from β – carbon atom then this elimination is known as β – elimination. There is also a simultaneous elimination of halogen atom from a – carbon. As a result, an alkene is formed.
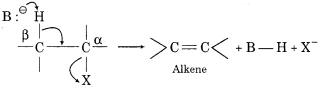
For Example:
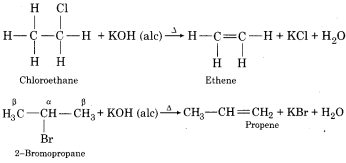
This reaction is also called dehydrohalogenation.
Question 6.
Write the mechanism of Carbyl amine reaction.
Answer:
Carbylamine Reaction or Isocyanide Reaction – When chloroform is heated with a primary amine and alcoholic KOH then isocyanide is formed with an extremely unpleasent smell which is also known as a carbyl amine. This reaction is used as a test for primary amine and chloroform, therefore, it is also known as isocyanide test.
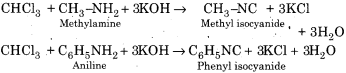
Mechanism:
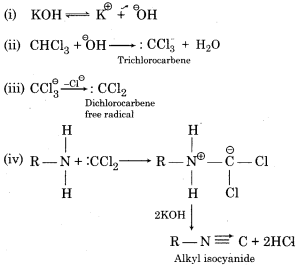
Question 7.
How will you prepare the following from chloroform:
(a) Acetylene
(b) CCl4
(c) Salicylaldehyde
Answer:
(a) Acetylene from Chloroform:
On warming with Ag powder, chloroform is converted into acetylene.
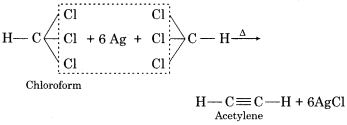
(b) CCl4 from Chloroform:
Chloroform on chlorination gives carbon tetrachloride.
![]()
(c) Salicylaldehyde from chloroform:
Chloroform and phenol are heated with alcoholic KOH to give salicylaldehyde.
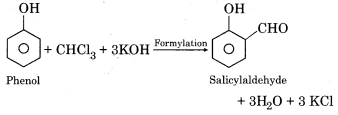
Question 8.
Write four uses of carbon tetrachloride.
Answer:
(i) It is used as a fire extinguisher.
(ii) It is used in dry cleaning.
(iii) It is used for the manufacture of freon and salicylic acid.
(iv) It is used as a solvent.
Question 9.
How will you prepare the following from aniline?
(a) Chlorobenzene
(b) Bromobenzene
(c) Iodobenzene
Answer:
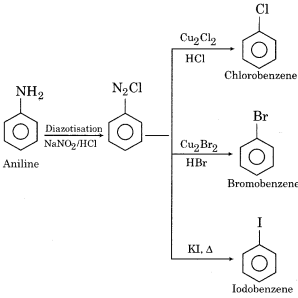
Question 10.
Write the formula of following:
(a) Freon – 11
(b) Freon – 12
(c) Freon – 111
Answer:
(a) Freon – 11 = CFCl3
(b) Freon – 12 = CF2Cl2
(c) Freon – 111 = C2FCl5
Question 11.
What happens when:
(i) Does ethyl bromide react with silver cyanide?
(ii) Iodoform is heated with silver power?
Answer:
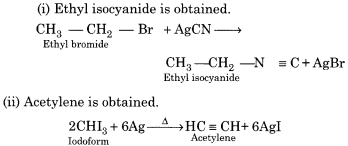
Question 12.
Benzyl chloride is more reactive than chlorobenzene. Why?
Answer:
Benzyl chloride is more reactive than chlorobenzene towards SN1 reaction because it readily ionises to give benzyl carbonation which is resonance stabilized.
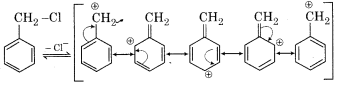
In chlorobenzene, ionisation leading to carbonation is not possible. Therefore it is less reactive.
RBSE Class 12 Chemistry Chapter 10 Long Answer Type Questions
Question 1.
Explain the following:
(a) Classification of halogen derivatives
(b) Nature of C-X bond in halogen derivatives.
(c) Directive influence of halogen atom in haloarene.
Answer:
(a) Classification of Halogen Derivatives:
In the IUPAC system of nomenclature, halogen derivatives are considered as halo-substituted derivatives of the respective alkane. The number of carbon atoms is used to consider the position of the halogen atom to which it is attached. The carbon atom carrying the halogen atom gets the lowest number. If two different halogen atoms present in halogen derivative then preference is given in both numbering and writing name to that halogen atom which comes first in the alphabetical order.
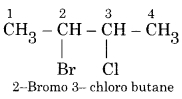
For example 2-Bromo- 3- chloro butane.
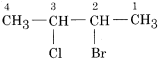
(i) These may be classified as mono, di, tri, tetra etc compounds depending on the number of halogen atoms present in haloalkane. For example,
| Formula | CH3 – Cl | CH2Cl2 | CHCl3 | CCl4 |
| Common Names | Methyl chloride | Methylene chloride | Chloroform | Carbon tetrachloride |
| IUPAC Name | Chloromethane | Dichloromethane | Trichloromethane | Tetrachloromethane |
| Series | Mono haloalkane | Dihalo alkane | Trihalo alkane | Tetra haloalkane |
(ii) Monohaloalkanes are classified into three types on the basis of nature of carbon atom to which halogen is attached. ‘
Primary Alkyl Halides:
In these compounds, the halogen atom is attached with a primary carbon atom.
For example:
| Formula | CH3 – Cl | CH3 – CH2 – Cl | CH3 – CH2 – CH2 – Cl |
| Common Name | Methyl chloride | Ethyl chloride | Propyl Chloride |
| IUPAC Name | Chloromethane | Chloroethane | 1 – Chloropropane |
Secondary Alkyl Halides:
In these halogen atoms is attached with a secondary carbon atom.
For example:
Tertiary Alkyl Halide:
In these compounds, the halogen atom is attached with the tertiary carbon atom.
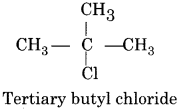
(iii) Allylic Halides:
In these compounds, the halogen atom is bonded to an sp3 hybridised carbon atom next to the C = C bond. The sp3 hybridised carbon is known as allylic carbon.
For example:
![]()
(iv) Benzylic Halides:
In these compounds, the halogen atom is bonded to an sp3 hybridised carbon atom next to an aromatic ring. This carbon is known as benzylic carbon.
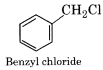
(v) Vinylic Halides:
In these compounds, the halogen atom is bonded to the sp2 – hybridised C – atom of a carbon-carbon double bond.
![]()
(vi) Aryl Halides or Haloarenes:
In these compounds, halogen is bonded to the sp2 – hybridised carbon atom of an aromatic ring.
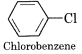
(b) Nature of C – X bond in halogen derivatives:
In haloalkanes, the formation of C – X bond takes place by the overlapping of sp2 – hybridised orbital of carbon and 3p (half filled orbital of chlorine (sp3– overlapping). This overlapping can be shown as:
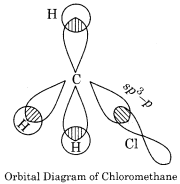
Although the C-X bond is the covalent bond value of electronegativity of C – atom is 2.6 which is less than electronegativity of the halogen atom. So due to the difference in electronegativities, the nature of C – X bond is polar. Therefore the halogen atom bears a partial negative charge whereas the carbon atom bears a partial positive charge.
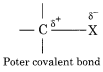
CH3 – Cl CHg – Br CH3 – I
H = 1.86D 1.79 D 1.62 D
(c) Directive Influence of Halogen atom in Haloarenes:
In haloarenes, the halogen atom present in the ring is ortho and para directing in nature and therefore in a substitution reaction, a mixture of isomeric products is formed, in which the para – isomer is the major product since the para position is less hindered in the molecule of haloarene compared to ortho position.
In haloarenes, the halogen atom has also a strong –I (inductive) effect which is likely to deactivate the ring and the electrophilic attack will become rather difficult. However, +M effect dominates over the –I effect when the electrophile is directed to ortho and para position in the ring. Therefore, the electrophilic substitution takes place at these positions though with difficulty. The electrophilic attack is not feasible at the meta position in the ring because the +M effect is rather weak and –I effect of the halogen atom is expressed to have greater magnitude.
Question 2.
How will you prepare:
(a) Alkyl halide from alcohol?
(b) Alkyl halide from halogen exchange?
(c) Chloroform from the acetone?
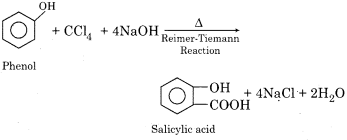
(d) Salicylic acid from carbon tetrachloride.
Answer:
(a) Alkyl halide from alcohol:
(i) By the reaction of alcohol and halogen acid.
R – OH + HCl \(\underrightarrow { ZnCL_{ 2 } }\) R – Cl + HCl
R – OH + HBr \(\underrightarrow { { H }_{ 2 }S{ O }_{ 4 } }\) R – Br + H2O
(ii) By the action of thionyl chloride on alcohol.
R – OH + SOCl2 \(\underrightarrow { { C }_{ 5 }{ H }_{ 5 }N }\) R – Cl + SO2 + HCl
(iii) By the action of phosphorus halides on alcohol.
R – OH +PCl5 \(\underrightarrow { \triangle } \) R – Cl +POCl3 + HCl
3R – OH +PCl3 \(\underrightarrow { \triangle } \) 3R – Cl + H3PO3
(b) Alkyl halide from halogen exchange:
In this reaction, alkyl halides are prepared from alkyl halides only.
For Example:
R – Cl + Nal \(\xrightarrow [ Acetone ]{ \triangle } \) R – I + NaCl
R – Br + Nal \(\xrightarrow [ Acetone ]{ \triangle } \) R – I + NaBr
This reaction is known as the Finkelstein reaction. The synthesis of alkyl fluoride is best accomplished by heating alkyl iodide in the presence of a metallic fluoride such as AgF. This reaction is called “Swarts reaction”.
R – I + AgF \(\rightarrow \) R -F + Agl
(c) Chloroform from Acetone:
![]()
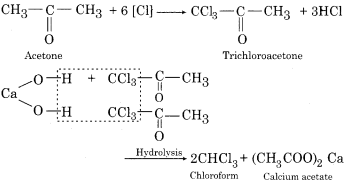 (d) Salicylic acid from carbon tetrachloride:
(d) Salicylic acid from carbon tetrachloride:
Question 3.
Write short notes on the following:
(a) Haloform Reaction
(b) Carbylamine Reaction
(c) Darzen’s Reaction
(d)Sandmeyer Reaction.
Answer:
(a) Haloform Reaction:
When a methyl ketone ![]() (even acetaldehyde) is reacted with halogen in aqueous sodium hydroxide, the ketone gets oxidised to the sodium salt of acid with one carbon less than ketone and at the same time haloform (CHX3) also gets formed.
(even acetaldehyde) is reacted with halogen in aqueous sodium hydroxide, the ketone gets oxidised to the sodium salt of acid with one carbon less than ketone and at the same time haloform (CHX3) also gets formed.
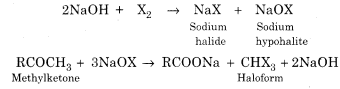
When the reaction is carried with iodine, a yellow precipitate of iodoform is obtained.
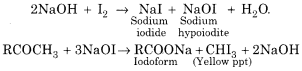
The iodoform test is quite useful to make a distinction between certain pairs of compounds one of which respond to the test while the other does not. For example:
(i) Methanal and ethanal (Test is given by ethanol)
(ii) Ethanal and propanal (Test is given by ethanol)
(iii) Pentan-2-one and pentane-3-one (Test is given by pentane – 2 – one)
(b) Carbylamine Reaction:
Chloroform is heated with primary aliphatic or aromatic amine and alcoholic KOH then it forms isocyanide or carbylamine with an extremely unpleasent smell. The reaction is not given by secondary and tertiary amines and is, therefore, a test for the primary amines.
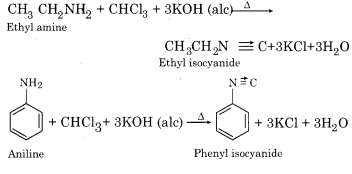
(c) Darzen’s Reaction:
It involves the reaction of straight chain primary alcohols with thionyl chloride to form chloro derivatives without rearrangement.
R – OH + SOCl2 \(\underrightarrow { Pyridine } \) RCl + SO2 + HCl
(d) Sandmeyer Reaction:
In this reaction, benzene diazonium chloride is reacted with CuCl or CuBr in the presence of corresponding halogen acid to form haloarene.
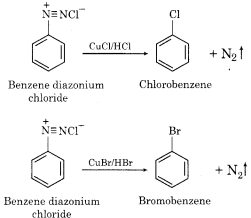
Question 4.
Explain the mechanism of SN1 and SN2
Answer:
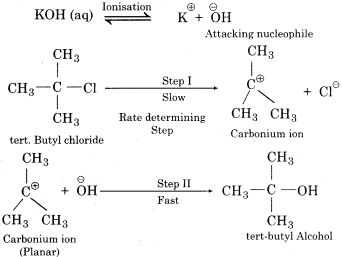
Unimolecular Nucleophilic Substitution or SN1
(i) These reactions proceed in two steps:
(ii) In step I:
Heterolytic cleavage of C-X forms a carbocation (carbonium ion) (R⊕) in the form of intermediate and a halide ion X–. Step I is the slowest step, therefore, it is rate determining step.
R-X \(\xrightarrow [ slow ]{ step } \) R⊕ + X–
(iii) In the first step, only alkyl halide is used therefore the rate of reaction depends only upon the concentration of alkyl halide.
Rate α [ R-X ]
Therefore, this reaction is known as unimolecular nucleophilic substitution reaction (SN1).
(iv) In the second step, carbocation is attacked by nucleophile to form a product. Rate of the second step is much more than the first step.

(v) Like the stability of carbocation formed in step I increases, the reaction will easily proceed. Order of stability of carbocation is as follows:
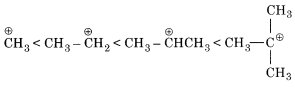
Order of reactivity of SN1 mechanism if halogen atom is the same.
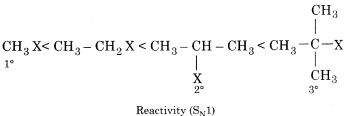
The intermediate carbocation is planar, therefore attack of the nucleophile may be accomplished from either side resulting in a mixture product. Therefore if the reactant is optical isomer then the product is a racemic mixture. Mechanism of SN1 reaction between tertiary butyl chloride and aqueous KOH can be explained in the following ways:
The overall reaction can be represented as follows:
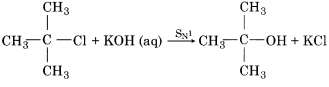
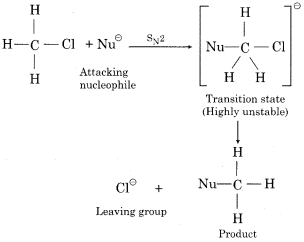
(b) Bimolecular Nucleophilic Substitution or SN2
(i) This type of reaction occurs in one step.
(ii) In this type of reaction the only formation of transition state occurs and no intermediate is formed.
(iii) For the formation of the transition state, the attacking nucleophile attacks from the opposite directions at from 180° angle of leaving the group (X®). This is known as backside attack or rear attack.
(iv) In the transition, state attacking nucleophile (Nu–) and leaving the group (X–) both are partially attacked with the central carbon atom.
(v) The rate of reaction depends upon the concentration of both the alkyl halide and the nucleophile. Therefore, these reactions are known as bimolecular nucleophilic substitution reaction (SN2).
Rate α [ R-X ] [ Nu– ]
(vi) In these reactions, the configuration of products is opposite to that reactants, therefore in these reactions inversion of configuration occurs. This is’ known as “Walden inversion”.
(vii) In these reactions, the presence of bulky alkyl group on the carbon of C-X bond produces steric hindrance for the attack of the nucleophile. Therefore with the increase in the number of alkyl groups on carbon, the reactivity of R-X decreases. If the halogen atom is same then the order of reactivity of alkyl halides towards SN2 mechanism is as:
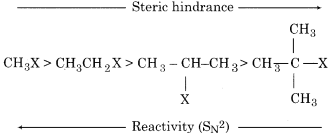
Therefore, primary alkyl halides react more rapidly by mechanism whereas tertiary alkyl halide reacts by the SN2 mechanism. Secondary alkyl halides will react with SN1and SN2 mechanism. This depends upon the nature of nucleophile and solvent.
Question 5.
Write a short note on the following?
(i) Freons
(ii) DDT
(iii) BHC
Answer:
(i) Freons:
Chlorofluoro derivatives of methane and ethane are called freons. They can be prepared by the reaction of HF with carbon tetrachloride (CCl4) or hexachloroethane in the presence of SbCl5.
For example:
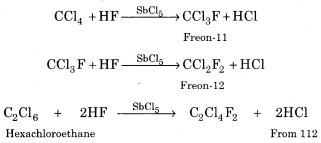
Properties of Freons:
Freons odourless, volatile liquids. They are highly inert and highly stable even at high temperature and pressure.
Uses of Freons
(i) They are used as inert solvents.
(ii) They are used as refrigerants in the refrigerator, air conditioners.
(iii) They are used in the form of propellant in aerosols.
(ii) DDT (Dichlorodiphenyltrichloroethane)
It is synthesized by treating a mixture of chloral with chlorobenzene in the presence of concentrated H2S04.
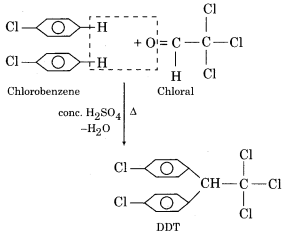
DDT is a white crystalline solid. It is used as an insecticide for killing or controlling mosquitoes, lice etc. DDT is a harmful poisonous substance and does not easily decompose.
(iii) B.H.C. (Benzene Hexachloride)
It has many common names like gammexane, lindane etc. Its IUPAC name is 1,2,3,4,5,6 – hexachlorocyclohexane. It is prepared by chlorination of benzene in the presence of UV radiations.
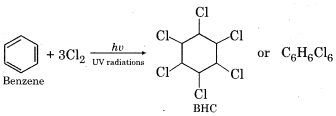
It is a mixture of many isomers (α, β, γ, δ, θ, η, ε, σ). BHC is used in an agricultural field in the form of insecticide. Insecticidal activity is highest in gamma-isomer (γ – BHQ), In comparison to other isomers, γ -isomer is comparatively small so its penetrating power is more.
Question 6.
Explain the electrophilic and nucleophilic reaction of chlorobenzene.
Answer:
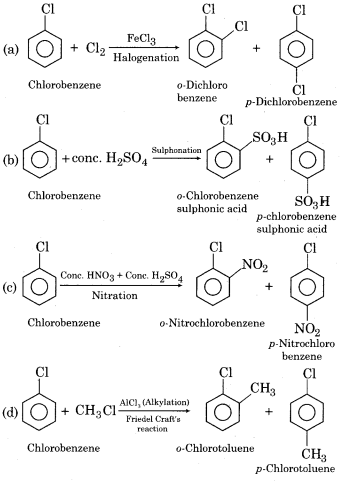
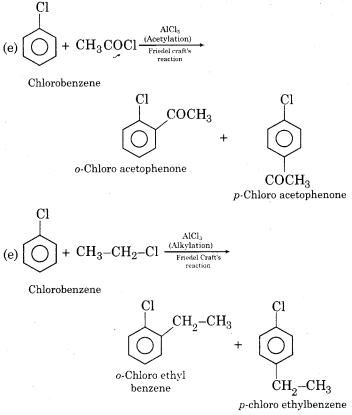
(i) Electrophilic substitution reactions of chlorobenzene:
In chlorobenzene, due to – I effect of a chlorine atom, electron density decreases on benzene ring and attack of electrophile occurs slowly. Due to this reason, chlorobenzene is less reactive towards electrophilic substitution reactions in comparison to benzene. Some electrophilic substitution reactions of chlorobenzene are as follows:
(ii) Nucleophilic Substitution Reaction of Chlorobenzene:
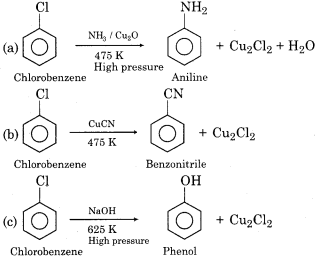
Due to resonance, there is a partial double bond character in the C-X bond of chlorobenzene, as a result, the bond cleavage becomes difficult. Due to this reason, chlorobenzene is less reactive towards nucleophilic substitution reaction than alkyl halides. However, nucleophilic substitution in chlorobenzene occurs under drastic conditions like high-temperature. Some nucleophilic substitution reactions of chlorobenzene are as follows:
Question 7.
How will you prepare the following from alkyl halide:
(i) Alkyl isocyanide
(ii) Alkyl cyanide
(iii) Nitroalkane
(iv) Alkyl nitrile
(v) Isopropylbenzene
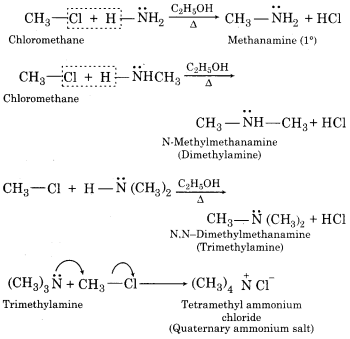
(vi) Tetramethylammonium chloride
Answer:
(i) When alkyl halide is treated with AgCN, alkyl isocyanide is obtained.
![]()
For example:
![]()
(ii) Alkyl halides react with an alcoholic solution of KCN to give alkyl cyanides.
![]()
For example:
![]()
(iii) When alkyl halide is treated with silver nitrate (AgNO2), nitroalkanes are obtained.
![]()
For example:
![]()
(iv) When an alkyl halide is treated with sodium or potassium nitrite, alkyl nitrite is obtained.
![]()
For example:
![]()
(v) When secondary alkyl halide (isopropyl chloride) is treated with benzene in the presence of anhydrous AlCl3 isopropylbenzene is obtained.
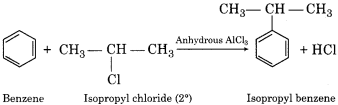
(vii) When alkyl halide (e.g. chloromethane) is heated with an alcoholic solution of ammonia in a sealed tube to about 373 K, a mixture of products containing primary, secondary, tertiary amines and quaternary ammonium salt is formed. This reaction is called Hoffmann ammonolysis reaction.