Rajasthan Board RBSE Class 12 Chemistry Chapter 3 Electrochemistry
RBSE Class 12 Chemistry Chapter 3 Text Book Type Questions
RBSE Class 12 Chemistry Chapter 3 Multiple Choice Questions
Question 1.
Which of the following is not a conductor?
(a) Cu(metal)
(b) NaCl(aq)
(c) NaCl(molten)
(d) NaCl(solid)
Question 2.
If conductance and conductivity of a cell are equivalent then cell constant will be :
(a) 1
(b) 0
(c) 10
(d) 1000
Question 3.
The unit of cell constant is
(a) ohm-1cm-1
(b) cm
(c) ohm-1 cm
(d) cm-1
Question 4.
The unit of conductivity (specific conductance) is:
(a) ohm-1
(b) ohm-1 cm-1
(c) ohm-1 cm2 equiv-1
(d) ohm-1 cm2
Question 5.
If redox reaction takes place in cell then electromotive force (e.m.f.) of cell will be:
(a) positive
(b) negative
(c) zero
(d) one
Question 6.
Which statement will be correct for the cell made up of zinc and copper on the basis of electro chemical series?
(a) Zinc will act as cathode and copper as anode.
(b) Zinc will act as anode and copper as cathode.
(c) The flow of electrons takes place from copper to zinc.
(d) Copper electrode dissolves and zinc is deposited at zinc electrode.
Question 7.
How many coulomb charge will required for oxidation of one mole H2O into O2
(a) 1.93 x 105 C
(b) 9.65 x 104 C
(c) 6.023 x 1023 C
(d) 4.825 x 104 C
Question 8.
Which layer is electroplated at iron sheet?
(a) C
(b) Cu
(c) Zn
(d) Ni
Question 9.
Which of the following mixture represents rusting of iron?
(a) FeO and Fe(OH)3
(b) FeO and Fe(OH)2
(c) Fe2O3 and Fe(OH)3
(d) Fe3O4 and Fe(OH)2
Question 10.
When lead storage cell discharge, then
(a) SO2 evolves
(b) Pb SO4 destroys
(c) Pb forms
(d) H2SO4 destroys
Answers:
1. (d) 2. (a) 3. (d) 4. (b) 5. (a) 6. (b) 7. (b) 8. (c) 9. (c) 10.(d)
RBSE Class 12 Chemistry Chapter 3 Very Short Answer Type Questions
Question 1.
Can you store copper sulphate solution in a zinc pot?
Answer:
Zinc is more reactive than copper. Hence it displaces copper from copper sulphate solution as follow:
Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
Thus, zinc reacts with CuSO4 solution. Hence we cannot store copper sulphate solution in a zinc pot. Alternatenely,
E°zn2+/Zn = 0.76 V
E°Cu2+ /Cu = 0.34 V
To check whether the reaction will take place or not, we have to find out EMF of this cell
Zn(s) + CuSO4 (aq) → ZnSO4 (aq) + Cu(s)
Cell Zn/Zn2+ || Cu2+/Cu
E°Cell = E°(Cathode) – E°(Anode)
= 0.34V – ( -0.76V)
E°Cell = + 1.10 V
As E°Cell is positive, so the reaction will take place. Hence we cannot store copper sulphate in a zinc pot.
Question 2.
Consult the table of the standard electrode potentials and suggest three substances that can oxidize ferrous ions under suitable conditions.
Answer:
For the oxidation of ferrous ion, following reaction will take place
Fe2+ → Fe3+ + e– E°ox = – 0.77 V
Only those sulstances can oxidise ferrous ion into ferric ion, which are stronger oxidising agents and have positive reduction potentials greater than 0.77 V so that EMF of the cell reeaction is positive. This is so for elements lying below Fe3+/Fe2+ in the electrochemical series e.g Br2, Cl2, and F2.
Question 3.
Why does the conductivity of solution decreases with dilution ?
Answer:
Conductivity of a solution is the conductance of ions present in a unit volume of the solution. On dilution, the number of ions per unit volume decreases. Hence the conductivity decreases.
Question 4.
Suggest a list of metals that are extracted electrolytically.
Answer:
Sodium, calcium, magnesium and aluminium.
Question 5.
Suggest two materials other than hydrogen that can be used as fuels in fuel cells.
Answer:
Methane and Methanol.
Question 6.
Arrange the following metals in the order in · which they displace each other from the solution of their salts. Ag, Al, Cu, Fe, Mg and Zn
Answer:
Mg, Al, Zn, Fe, Cu, Ag.
RBSE Class 12 Chemistry Chapter 3 Short Answer Type Questions
Question 1.
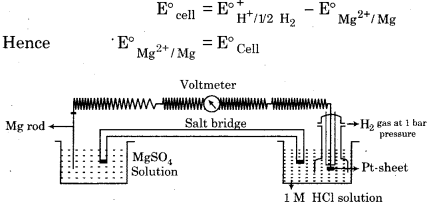
How would you determine the standard electrode potential of Mg2+/Mg?
Answer:
For determing the standard electrode potential of Mg2+/Mg, we have to set up a cell in which one electrode is Mg/Mg SO4 (1 M), can be made by dipping a magnesium wire in 1 M MgSO4 solution and the other one is hydrogen electrode Pt, H2 (1 atm)/H+ (1 M). Now measure the e.m.f. of the cell. Here we have to note also the direction of deflection in the voltmeter. The direction of deflection shows that electrons flow from magnesium electrode to hydrogen electrode i.e., oxidation takes place on magnesium electrode and reduction on hydrogen electrode. Hence the cell may be represented as,
Mg/Mg2+ (1 M) || H+ (1 M)/H2(1 atm), Pt
Question 2.
Calculate the potential of hydrogen electrode in contact with a solution whose pH is 10.
Answer:
For, pH = 10
pH = log [H+]
[H+] = 10-pH
[H+] = 10-10 mol L-1
For hydrogen electrode,
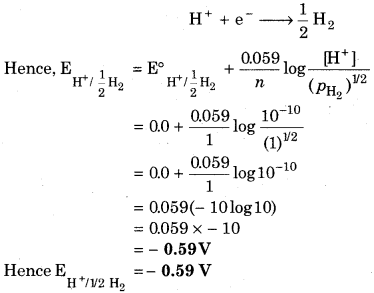
Question 3.
Calculate the e.m.f. of the cell in which the following reaction takes place :
Ni(s) + 2Ag+ (0.002 M) → Ni2+ (0.160 M) + 2Ag (s) Givent that E°Cell = 1.05 V
Answer:
Given that
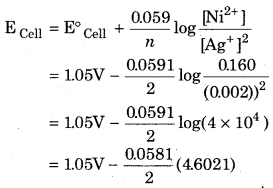
= 1.05V – 0.134V = 0.916 V
Question 4.
The cell in which the following reaction occurs:
2 Fe3+(aq) + 2I–(aq) → 2 Fe2+(aq) + I2(s) has E°Cell = 0.236 V at 298 K. Calculate the standard Gibb’s energy and the equilibrium constant for the cell reaction.
Answer:
2 Fe3+ + 2e– → 2 Fe 2+
2I– → I2 + 2e–
Hence for the given cell reaction n = 2
∆rGo = – nFE°Cell
= – 2 x 96500 x 0.236J
= – 45.55 kJ mol-1
∆rGo = – 2.303 RT log Kc
![]()
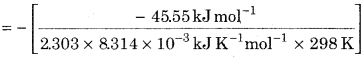
= 7.983
Kc = Antilog (7.983)
= 9.616x 107
Question 5.
Suggest a way to determine the ∧°m( HCl ) – ∧°m( NaCl )
Answer:
At infinite dilution, the limiting molar concentration of water ∧°m( H2O ) can be calculated by knowing the molar concentration of sodium hydroxide, hydrochloric acid and sodium chloride at infinite dilution.
∧°m(H2O) = ∧°m(HCl) + ∧°m(NaOH) – ∧°m(NaCl)
Question 6.
The molar conductivity of 0.025 mol L-1 methanoic acid is 46.1 S cm2 mol-1. Calculate its degree of dissoiation and dissociation constant. Given ∧°m(H+) = 349.6 S cm2 mol-1 and ∧°m(HCOO–) = 546 S cm2 mol-1?
Answer:
∧°m (HCOOH–)= ∧°m (H+) + ∧°m (HCOO–)
= 349.6 + 54.6
= 404.2 S cm2 mol-1
Question 7.
Consider the reaction
Cr2O72- + 14H+ + 6e– → 2Cr3+ + 7H2O
What is the quantity of electricity in coulombs needed to reduce 1 mol of Cr2O72-
Answer:
According to equation for the reduction of one mole of Cr2O72- , 6 mole of electrons are needed. Hence the amount of electricity require = 6F
= 6 x 96500 C = 579000 C
Question 8.
Write the chemistry of recharging the lead storage battery, highlighting all the materials that are involved during
recharging.
Answer:
Lead storage battery can be recharged by passing electric current of suitable patential in opposite direction in which lead is again deposited at anode and PbO2 is deposited at cathode. The density of electrolyle i.e., H2SO4 again increases. The following cell reaction takes place during charging :
At Anode : Pb(s) + SO4-2(aq) → PbSO4(s) + 2e–
At Cathode : PbO2(s) + SO-2(aq) + 4H+(aq) + 2e– → PbSO4(s) + 2H2O(l)
Overall reaction : Pb(s) + PbO2(s) + 2H2SO4(aq) → 2PbSO4(s) + 2H2O(l)
Question 9.
Given the stardard electrode potentials
K+/K = – 2.93 V, Ag+/Ag = 0.80 V
Hg22+/Hg = 0.79V, Mg2+/Mg = – 237V, Cr2+/Cr = – 0.74 V
Arrange these metals in their increasing order of reducing power.
Answer:
Higher the oxidation potential, more easily it is oxidized and hence greater is the reducing power. Thus increasing order of reducing power will be Ag < Hg < Cr < Mg
Question 10.
Calculate the standard cell polentials of galvenic cells in which the following reactions take place :
(i) 2Cr(s) + 3Cd2+(aq) → 2Cr3+(aq) + 3Cd(s)
(ii) Fe2+(aq) + Ag+(aq) → Fe3+(aq) + Ag(s)
Given
E°Cr3+/Cr = – 0.74 V, E°Cd2+/Cd = 0.40 V
E°Ag+/Ag = 0.80 V, E°Fe3+/Fe2+ = 0.77 V
Also calculate ArGo and equlibrium constants for the reactions.
Answer:
(1) 2Cr (s) + 3Cd(aq)2+ → 2Cr3 + 3Cd(s)
E°cell = E°Cathode – E°Anode
= E°(Cd2+cd) – E°(Cr3+/Cr)
= – 0.40V – (-0.74V)
= – 0.40 V + 0.74V
= + 0.34V
∆G° = -nFE°Cell
= – 6 mol x 96500 C mol-1 x 0.34 V
= – 196860 CV mol-1
= – 196.860 J mol-1
∆G° = – 2.303RT log Kc
– 196.860 kJ = – 2.303 x 8.314 x 298 x logKc
![]()
log Kc = 34.50/4
Kc= Antilog 34.50/4
= 3.173 x 1034
Hence Gibb’s energy of cell (∆G°)= – 196.86 kJ mol
Equilibrium constant (Kc) = 3.173 x 1034
(ii) Fe2+(aq) + Ag+(aq) → Fe3+(aq) + Ag(s)
E°Cell = E°(Cathode) – E°(Anode)
= E°(Ag+/ Ag) – E°(Fe3+, Fe)
= + 0.80 V – 0.77V
= 0.03V
∆G° = – n F E°Cell
= – 1 mol-1 x 96500 C mol-1 x 0.03
= – 2895 J mol-1
= – 2.895 kJ mol-1
∆G° = – 2.303 RT log Kc
– 2895 = – 2.303 RT log Kc
– 2895 = – 2.303 x 8314 x 298 x logKc
![]()
logKc = 0.5074
Kc = Antilog 0.5074
Kc = 3.22
Gibb’s energy of cell = 2.895 kJ mol-1
Equilibrium constant of cell = 3.22
RBSE Class 12 Chemistry Chapter 3 Long Answer Type Questions
Question 1.
Explain how rusting of iron is considered as setting up of an electrochemical cell.
Answer:
In rainy season and in highly humid atmosphere the iron surface has a layer of water. This water layer dissolves acidic oxides of the air like CO2, SO2 etc. to form acids which dissociate to give H+ ions.
H2O + CO2 → H2CO3 ⇌ 2H+ + CO32+
In the presence of H+ ions, iron starts losing electrons at some spot to form ferrous ions, i.e., its oxidiation takes place. Here it is anode.
Fe(s) → Fe2+(aq) + 2e–(anode)
Now the released electrons move through the metal and reach to another spot where H+ ions and the dissolved oxygen take up these electrons and reduction reaction takes place. Here it is cathode.
O2(g) + 4H+(aq) + 4e– +2H2O(l)(cathode)
The overall reaction is
2Fe(s) + O2(g) + 4H+(aq) → 2Fe2+(aq) + 2H2O(l)
Thus in this way an electrochemical cell is set up on the surface. This ferrous ion further oxidised by the atmospheric oxygen to ferric ions which combine with water molecules to from hydrated ferric oxide,
Fe2O3 x H2O, which is rust.
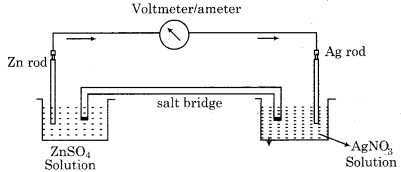
Question 2.
Depict the galvenic cell in which the reaction
Zn(s) + 2Ag+(aq) → Zn2+(aq) + 2Ag(s)
takes place. Further, show,
(i) Which of the electrodes is negatively charged?
(ii) The carriers of the current in the cell.
(iii) Individual reaction at each electrode.
Answer:
The given chemical reaction is as follows
Zn(s) + 2Ag+(aq) → Zn2+(aq) + 2Ag(s)
Cell can be represented as,
Zn(s)/Zn2+(aq) || Ag+(aq)/Ag(s)
(i) The electrode at which oxidation takes place, behaves as anode and it is negatively charged. Hence zinc electrode is negatively charged.
(ii) In the cell, the carriers of the current are electrons. and the current will flow from silver to copper in the external circuit.
(iii) The reactions on each electrode are as follows.
At anode : Zn(s) → Zn2+(aq) + 2e–
At cathode : Ag+(aq) + e– → Ag(s)
It can be represented by the following diagram