RBSE Solutions for Class 9 Science Chapter 3 Atomic Structure are part of RBSE Solutions for Class 9 Science. Here we have given Rajasthan Board RBSE Class 9 Science Chapter 3 Atomic Structure.
| Board | RBSE |
| Textbook | SIERT, Rajasthan |
| Class | Class 9 |
| Subject | Science |
| Chapter | Chapter 3 |
| Chapter Name | Atomic Structure |
| Number of Questions Solved | 85 |
| Category | RBSE Solutions |
Rajasthan Board RBSE Class 9 Science Chapter 3 Atomic Structure
Atomic Structure Textbook Questions Solved
Objective Type Questions
Question 1.
Plum pudding model of the atom was proposed by:
(A) Neils Bohr
(B) Thomson
(C) Rutherford
(D) Goldstein James
Answer: B
Question 2.
Neutron was discovered by:
(A) C.V. Raman
(B) Rutherford
(C) J.J. Thomson
(D) Chadwick 10-8 cm
Answer: D
Question 3.
The size of an atom is:
(A) 10-6cm
(B) 10-5cm
(C) 10-2 cm
(D) 10-8 cm
Answer: D
Question 4.
The number of neutrons in deuterium, an isotope of hydrogen is:
(A) one
(B) two
(C) three
(D) None
Answer: A
Atomic Structure Very Short Answer Type Questions
Question 5.
Define isotopes.
Answer
Isotopes: Atoms of the same element having the same atomic number but different mass numbers are called isotopes.
For example, Chlorine has two isotopes
\(^{ 35 }{ Cl }\quad and\quad _{ 17 }^{ 37 }{ Cl } \), in which atomic number is 17 for both, but mass numbers are 35 and 37 respectively.
Question 6.
Define isobars.
Answer
Isobars: Atoms of different elements having the same mass number, but different atomic numbers are known as isobars.
For example, Calcium and argon have the same mass number 40, but different atomic numbers 20 and 18, respectively.
Question 7.
What are the fundamental particles of an atom?
Answer
Electron, proton, and neutron are called fundamental particles of an atom.
Question 8.
Define atomic mass.
Answer
Atomic mass (or atomic weight) of an element is defined as the relative mass of the atom of the element as compared to the mass of an atom of C-12 isotope, i.e
\( Atomic mass (or Atomic Weight)=\cfrac { Mass\quad (or\quad Weight\quad of\quad the\quad element) }{ \cfrac { 1 }{ 12 } th\quad of\quad mass\quad of\quad C-12\quad isotope } \)
Question 9.
Define atomic number.
Answer
Atomic number: The number of unit positive charges (or protons) present in the nucleus of an atom of a particular element is called the atomic number of that element. Furthermore, the number of protons is exactly equal to the number of electrons present in a neutral atom. Atomic number is represented by Z. Thus Atomic Number (Z) = Number of Protons = Number of Electrons, in a neutral atom The atomic number is represented by putting ‘Z’ in subscript on the left-hand side of the symbol of the element, for e.g., 2He for helium as He has only two protons in its nucleus. Similarly, for the atomic number of potassium is 19, it means that the potassium atom has 19 protons and 19 electrons in its neutral atom and is depicted by K.
Question 10.
What is the charge on neutron?
Answer
Neutron carries no charge, i.e., the neutron is a neutral particle.
Question 11.
Write the value of Avogadro’s number.
Answer
The value of Avogadro’s number is 6.022 x 1023.
Question 12.
Who discovered proton?
Answer
Proton was discovered by Goldstein.
Atomic Structure Short Answer Type Questions
Question 13.
What is an electric discharge tube? Explain with a diagram.
Answer
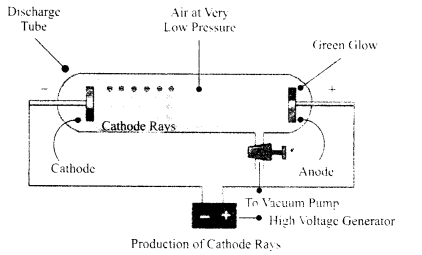
Electrical discharge tube:
Electrical discharge through gases is studied by using a specially designed glass tube commonly called a discharge tube. It consists of a cylindrical glass tube having a side tube, and two metal electrodes, one at each end. These electrodes can be connected to the respective terminals of a high tension power supply. The air inside the tube can be pumped out by connecting the side tube to a vacuum pump, and the desired pressure can be maintained inside the tube.
Question 14.
Explain the postulates of Thomson’s model of an atom.
Answer
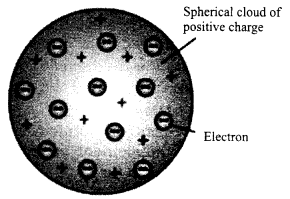
Thomson suggested that an atom is positively charged sphere of radius 10-8 cm. Negatively charged electrons are embedded in the sphere like plums in the pudding, or like the seeds in the edible red interior of a watermelon.
Since the two types of charges are equal in amount, the atom on the whole electrically neutral. It is also known as the plum-pudding model.
Question 15.
Explain the mole concept.
Answer
Wilhelm Ostwald introduced the term mole (Latin ‘moles’ means heap), in 1896 which was accepted as a unit to represent a large number of atoms in 1967. The Mole is the amount of a substance which contains as many particles as in 12g of C-12. Thus, one mole of any species is that quantity in number which has a mass equal to its atomic or molecular mass in grams.
For example, 1 mole of carbon isotope (atomic mass = 12) is equal to 12g.
1 mole of oxygen (Oa, molecular mass = 2 x 16) is equal to 32 g.
1 mole of water (H2O, molecular mass = 2×1 + 1×16) is equal to 18 g.
Question 16.
Write the main postulates of Dalton’s atomic theory.
Answer
The main postulates of Dalton’s atomic theory are:
- Every matter is made up of very small particles, called the atoms.
- Atoms are indivisible particles which can neither be created nor destroyed, in a chemical reaction.
- Atoms of a given element are identical in mass as well as in chemical properties.
- Atoms of different elements have different masses and chemical properties.
- Atoms combine in the ratio of small whole numbers to form compounds.
- The relative numbers, as well as the kind of atoms, are constant in a given compound.
Question 17.
Write the characteristics of Cathode rays.
Answer
The characteristics of Cathode rays are:
- When these rays strike the glass walls of the tube, a greenish glow is produced.
- When an electric field is applied through metal plates, placed over the discharge tube, cathode rays are deflected towards the positive terminal, showing that cathode rays consist of negatively charged particles.
- When a strong magnetic field is applied, cathode rays are deflected which shows cathode rays consist of moving charged particles.
- If a solid is placed in their path, its shadow is observed on a screen, showing that cathode rays move in a straight line.
- When a wheel is placed in their path, it starts moving, showing that cathode rays consist of material particles, possessing kinetic energy.
Question 18.
Explain covalent radius of an atom with an example.
Answer
Covalent radius is the half of the distance between the nuclei of two covalently bonded atoms of the same element in a molecule. Example, Covalent radius of chlorine molecules is 99Å.
Atomic Structure Long Answer Type Questions
Question 19.
Describe Rutherford’s nuclear model of an atom with the help of the gold foil experiment and well-labeled diagram.
Answer
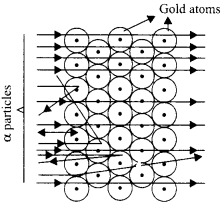
Rutherford designed an experiment in which fast-moving alpha-particles were made to fall on a thin gold foil. On the basis of the experiment, Rutherford proposed a model of an atom as follows:
(a) There is a positively charged region located at the center in an atom called the nucleus. Nearly, all the mass of an atom resides in the nucleus.
(b)The electrons revolve around the nucleus in well-defined orbits.
(c) The size of the nucleus is very small as compared to the size of the atom.
Question 20.
Describe the main postulates of the Neils Bohr model of the atom. Draw and explain the atomic structure of Na and K based on it.
Answer
Bohr’s model of an atom:
1. An atom is made up of charged particles, electrons, and protons. Electrons are negatively charged, whereas protons are positively charged.
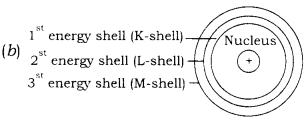
2. The electrons revolve around the nucleus in fixed orbits called energy levels or shells which are represented by numbers 1, 2, 3, — or letters K, L, M, N, — counted from the nucleus outwards.
3. Protons are present within the nucleus. Due to the presence of protons, the nucleus is positively charged.
4. Each energy level has a fixed amount of energy. The energy of orbits increases with distance from the nucleus.
5. The number of electrons and protons are equal so that an atom, on the whole, is electrically neutral.
6. There is no change in the energy of electrons as long as they revolve in the same orbit and remain stable. However, if an electron gains energy, it jumps to a higher energy level and if it loses energy, it falls back to the lower energy level.
Structure of potassium: The atomic number of potassium is 19 and its mass the number is 39. It is represented by the symbol \( { 19 }^{ 39 }{ K } \)
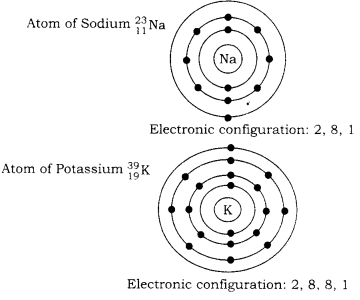
Being a neutral atom, it has: Number of electrons = 19 = Number of protons & Number of neutrons = 39 – 19 = 20 The nucleus of potassium contains 19 protons and 20 neutrons i.e. 39 nucleons.
According to rule 1, K – shell (n = 1) can not have more than 2 electrons. L-shell according to rule 1
(n = 2) can not have more than 8 electrons. The new shell i.e. the M-shell begins to fill and may contain a maximum of 18 electrons. However, being the outermost shell, it can not have more than 8 electrons (Rule 2). Thus, no. of electrons in N-shell = 8.
Now, according to rule 4, the filling of N-shell starts and since only one electron is left after filling K, L, M shell it occupies N-shell.
Thus, the electronic configuration of potassium would be 2, 8, 8, 1. or K – shell = 2 electrons, L – shell = 8 electrons M – shell = 8 electrons, N – shell = 1 electron Structure of Sodium: Same rule applies for sodium element also. Thus, the electronic configuration of sodium would be 2, 8, 1 K shell = 2 electron L shell = 8 electron M shell = 1 electron.
Question 21.
What are positive rays? How are they produced? Write their properties also.
Answer
While working with cathode rays, Sir J.J. Thomson had noticed a red glow around the cathode, on the opposite side of the anode. To know the reason for this glow, he designed a discharge tube with a perforated cathode fixed in the middle of the tube, as shown in the figure.
On applying an electrical potential across the two electrodes, green fluorescence was seen in the glass tube at one end, while on the other end, red fluorescence was seen. The green fluorescence was due to the bombardment of glass by the cathode rays. Obviously, the red fluorescence was due to some other type of rays. J.J. Thomson later showed that these rays causing red fluorescence consisted of positively charged particles and were thus, named as positive or anode rays. These rays were also called canal rays.
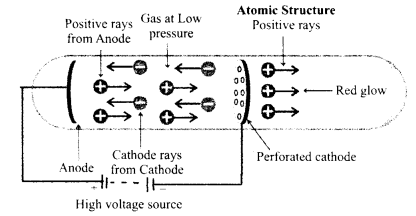
Production of positive rays: Cathode rays consist of negatively charged particles called electrons. These electrons move away from the cathode with very high speeds. These fast-moving electrons split the molecule into atoms, and remove one or more electrons from the atoms. Thus, the atoms get converted into the positive ions due to the loss of electrons. These positive ions pass through the holes in the cathode plate to produce a glow on the glass wall of the discharge tube. A stream of these positively charged particles is called positive rays or anode rays.
Properties of positive rays:
(a) Positive rays consist of positively charged particles.
(b) The nature of these rays depends on the gas used, in the discharge tube.
(c) These rays travel in straight lines.
(d) These rays get deflected by an electrical field and bend towards the negative plate. Thus, the deflection of the positive rays is in a direction opposite to that shown by the cathode rays.
(e) These rays are also deflected by the magnetic fields, in a direction opposite to that of the cathode rays.
(f) These rays can produce mechanical as well as chemical effects.
(g)The ratio of charge (e) to mass (m), i.e., (e/m) for the particles in the positive rays is not the same for all gases.
(h)The ratio e/m for the positive rays is very low as compared to the e/m value for cathode rays. This shows that the particles in the positive rays are heavier than the particles in the cathode rays.
Numerical Questions Solved
Question 1.
An isotope of an element having a number of neutrons 9 and its mass number is 17. Write the name and an atomic number of the element.
Answer
Mass number = Z + n
17 = Z + 9
Z = 17 – 9
Z = 8
Atomic number = 8 The name of the element is oxygen.
Question 2.
Calculate the weight of nitrogen (in gms) at 22.4 liters at NTP.
Answer
At NTP, one mole of a gas occupies a volume of 22.4 liters. Since, N, molecules have
molecular weight = 28 grams.
Thus, 22.4 liters of N0 at NTP has weight = 28 grams.
Question 3.
How many numbers of atoms of C is present in 1.5 moles of carbon?
Answer
1 mole of C = 6.022 x 1023 atoms
1.5 mole of C = 6.022 x 10a x 1.5 = 9.033 x 1023 atoms of carbon.
Question 4.
How many water molecules are present in 9 gm of water?
Answer
We know that 1 mole of any compound contains 6.022>< 1023 molecules of that compound.
Here, mass of water = 9 gm
Molar mass of water (H2O) = (2 x 1 + 1 x 16)g/mol = 18g/mol
∵ 18 g (= 1 mol of H2O contains 6.02 x 1023 molecules.
∴ 9 gm of H2O contains \( = [latex]\cfrac { 6.022\times { 10 }^{ 23 }\times 9g }{ 18g } =3.011\times { 10 }^{ 23 } molecules \)
Atomic Structure Additional Questions Solved
I. Multiple Choice Questions (MCQs)
Question 1.
The accepted unit of atomic and molecular masses is:
(A) kilogram
(B) gram
(C) pound
(D) atomic mass unit
Answer: D
Question 2.
An element whose gram-atomic mass and gram-molecular mass are the same is:
(A) hydrogen
(B) oxygen
(C) nitrogen
(D) helium
Answer: D
Question 3.
Mass of Avogadro’s number of oxygen (O) atoms is equal to:
(A) 16 u
(B) 16 g
(C) 32 g
(D) 6 kg
Answer: B
Question 4.
The mass percentage of hydrogen (H) in water (H2O) is:
(A) 1.11
(B) 88.9
(C) 8.89
(D) 11.11
Answer: D
Question 5.
The number of molecules in 8 g of oxygen gas is:
(A) 6.02 x 1023
(B) 3.01 x 1023
(C) 1.5 x 1023
(D) 3.01 x 1012
Answer: C
Question 6.
Which of the following is used as the ‘reference standard’ used at present, for describing the atomic and molecular masses?
(A) Carbon-13
(B) Chlorine-35
(C) Oxygen-8
(D) Carbon-12
Answer: D
Question 7.
The gas which has a molecular mass twice that of oxygen gas is:
(A) CO2
(B) CO
(C) SO2
(D) H2S
Answer: C
Question 8.
The element which can form gases having different atomicity is:
(A) chlorine
(B) oxygen
(C) hydrogen
(D) nitrogen
Answer: D
Question 9.
A number of elements present in ammonium phosphate are:
(A) 2
(B) 3
(C) 4
(D) 5
Answer: C
Question 10.
If atomic mass of nitrogen is 14 u and atomic mass of hydrogen is 1 u, what is the molar mass of ammonia?
(A) 17 u
(B) 15 u
(C) 34 u
(D) 30 u
Answer: B
Question 11.
Molar mass of HNO3 is 63 u, and an atomic mass of hydrogen and nitrogen are 1 u and 14 u respectively. Atomic mass of oxygen will be:
(A) 3 u
(B) 16 u
(C) 48 u
(D) 24 u
Answer: C
Question 12.
One mole of BaCf ionises completely, total moles of barium and chloride ions will be:
(A) 1 mole
(B) 2 moles
(C) 3 moles
(D) 02 x 1023 moles
Answer: A
Question 13.
A neutral atom contains an equal number of………
(A) Protons and Electrons
(B) Protons and Neutrons
(C) Neutrons and Electrons
(D) Protons, Electrons, and Neutrons
Answer: A
Question 14.
Particles present in the nucleus are:
(A) Proton and Electron
(B) Proton and Neutron
(C) Neutron and Electron
(D) Only Neutron
Answer: B
Question 15.
The presence of the nucleus at the centre of the atom was suggested by:
(A) J.J. Thomson
(B) Rutherford
(C) Goldstein
(D) Bohr
Answer: A
Question 16.
A maximum number of electrons which can be accommodated in the nth energy level is given by:
(A) n2
(B) 2n2
(C) 2n
(D) n
Answer: B
Question 17.
The fundamental particles with proper electric charge in an atom are:
(A) Electron (- 1), Proton (+ 1) and Neutron (1)
(B) Electron (- 1), Proton (0) and Neutron (0)
(C) Electron (+ 1), Proton (0) and Neutron (- 1)
(D) Electron (- 1), Proton (+ 1) and Neutron (0)
Answer: D
Question 18.
The correct electronic configuration of sodium atom is:
(A) 2, 8
(B) 2, 8, 1
(C) 8, 2, 1
(D) 1, 2, 8
Answer: B
Question 19.
Which of the following is correct about the energy of the shells in an atom?
(A) M > L > K
(B) M < L < K
(C) K > M > L
(D) M > K > L
Answer: A
Question 20.
An atom of an element with atomic number 18 and mass number 40 has the following articles:
(A) 18 protons, 18 electrons, 22 neutrons
(B) 18 protons, 22 electrons, 18 neutrons
(C) 22 protons, 18 electrons, 18 neutrons
(D) 22 protons, 22 electrons, 18 neutrons
Answer: A
Question 21.
Rutherford’s scattering experiment of resulted in the discovery of:
(A) electron
(B) proton
(C) neutron
(D) nucleus in an atom
Answer: D
Question 22.
A mass number of an atom is equal to the:
(A) number of protons in the nucleus of the atom
(B) number of electrons and protons in the atom
(C) number of neutrons and protons in the nucleus of an atom
(D) number of neutrons in the nucleus of an atom
Answer: C
Question 23.
A number of electrons in the valence shell of aluminum atom (at. no. 13) is:
(A) 1
(B) 2
(C) 3
(D) 0
Answer: A
Question 24.
Which of the following pairs represents a pair of isotopes?
(A) 8p + 8n ; 8p + 9n
(B) 9p + 9n ; 8p + 9n
(C) 18p + 22n ;16p + 24n
(D) 9p + 9n ; 8p + 10n
Answer: A
Question 25.
The number of electrons in an element X is 15 and the number of neutrons is 15 and the number of neutrons is 16. Which of the following is the correct representation of the element?
(A) \( _{ 15 }^{ 31 }{ X } \)
(B) \( _{ 16 }^{ 31 }{ X } \)
(C) \( _{ 16 }^{ 15 }{ X } \)
(D) \( _{ 15 }^{ 16 }{ X } \)
Answer: A
Question 26.
The ion of an element has 3 unit positive charge. A mass number of its atom is 27 and number of neutrons in it is 14. What is the number of electrons in the ion?
(A) 14
(B) 13
(C) 27
(D) 10
Answer: D
Question 27.
The mass number of an element is 7 and number of neutrons in its atom is 4. What is the number of electrons in the atom?
(A) 3
(B) 4
(C) 7
(D) 11
Answer: A
Question 28.
Which of the following configuration is not possible as per the Bohr’s model of an atom?
(A) 2, 5
(B) 2, 8, 8
(C) 2, 7, 3
(D) 2, 8, 2
Answer: C
Question 29.
Which of the following pairs represents a pair of isobars?
(A) 8p + 8n ; 8p + 9n
(B) 18p + 22n ; 20p + 20n
(C) 18p + 22n ; 16p + 22n
(D) 8p + 9n ; 8p + 8n
Answer: B
Atomic Structure Very Short Answer Type Questions
Question 1.
Define the term atom.
Answer
An atom is the smallest particle of an element that can take part in a chemical reaction. It may or may not exist independently.
Question 2.
Define law of conservation of mass for a chemical reaction.
Answer
Law of conservation of mass states that “mass can neither be created nor destroyed in a chemical reaction”.
Question 3.
State the law of constant proportion.
Answer
Law of Constant Proportion: In a chemical substance, the elements are always present in a definite proportion by mass.
Question 4.
‘Atoms of most elements are not able to exist independently. ’Name two atoms which exist as independent atoms.
Answer
Noble gases such as argon (Ar), Helium (He) exist as independent atoms.
Question 5.
Define atomicity.
Answer
The number of atoms present in one molecule of an element or a compound is known as its atomicity.
Question 6.
Give one relevant reason why scientists, initially choose 1/16 of the mass of an atom of naturally occurring oxygen as the atomic mass unit.
Answer
Initially, 1/16th of the mass of naturally occurring oxygen was taken as the atomic mass unit because this unit gave masses of most of the elements as whole numbers.
Question 7.
What is molar mass? What are its units?
Answer
The mass of one mole of a substance is called its molar mass. Its unit is gram per mole (g/mol.)
Question 8.
How many moles of CO2 are there is 11g of carbon dioxide? Molar mass of CO2 is 44 g/mol.
Answer
![]()
Question 9.
Name the scientists who proposed the law of conservation of mass and law of constant proportions.
Ans.
Lavoisier proposed the law of conservation of mass and Proust proposed the law of constant proportions.
Question 10.
Which two fundamental particles are present inside the nucleus?
Answer
Protons and neutrons.
Question 11.
Which particles were bombarded by Rutherford on a very thin gold foil?
Answer
α-particles.
Question 12.
Chemical properties of all the isotopes of an element are similar. Give one reason.
Answer
Isotopes of an element have similar electronic configuration and the same number of valence electrons.
Question 13.
What is Orbit?
Answer
According to Bohr’s model, the electron in an atom moves around the nucleus in a fixed circular path. This path is called orbit.
Question 14.
An element has mass number 28 and atomic number 14. Find the number of protons, neutrons, and electrons in it. Write the complete symbol of the element.
Answer
Number of protons =14
Number of neutrons =14
Number of electrons =14
Symbol of the element is \( _{ 14 }^{ 28 }{ Si } \)
Question 15.
If the atomic number of an element is 11 and its mass number is 23, then what is the number of neutrons in its nucleus?
Answer
Atomic mass of sodium atom is 23 and its atomic number is 11.
Hence a number of neutrons (N) will be 23 – 11 = 12.
Atomic Structure Short Answer Type Questions
Question 1.
Why do the canal rays obtained by using different gases have different e/m values?
Answer
When the electrons emitted from the cathode collide with the neutral atoms of the gas present in the tube, they remove one or more electrons present in them. This leaves behind positively charged particles, which travel towards the cathode. As the atoms of different gases have a different number of protons present in them. Therefore, different gases are found to have different e/m values.
Question 2.
What happens when the cathode rays are passed through an electric field between two parallel plates? Can one determine the nature of the charge of the particles constituting the cathode rays from this experiment? If Yes, how?
Answer
When the cathode rays are passed through an electric field, they are deflected towards the positively charged plate. This deflection of cathode rays towards the positively charged electrode indicated that the particles in the cathode rays are negatively charged particles.
Question 3.
The electronic configuration of an element X is 2, 8, 7.
1. What is the atomic number of the element X?
2. Is X metal or non-metal?
3. What is the valency of X?
Answer
1. Z = 17
2. Non-metal
3. 1.
Question 4.
If an atom carries one proton and one electron, will it carry ant) next charge or not?
Answer
This atom will not carry any next charge, because the positive charge of a proton will be balanced by the negative charge of the electron.
Question 5.
What is the difference between isobar: and isotopes ? Give one example for each
| Isobars | Isotopes |
|
1. They have a same mass number but different atomic numbers. 2. They have different numbers of protons. 3. Example: \(_{ 20 }^{ 40 }{ A }\quad and\quad _{ 18 }^{ 40 }B\quad \) |
1. They have different mass numbers but the same atomic number. 2. They have the same number of protons. 3. Example: \(_{ 92 }^{ 238 }{ A }\quad and\quad _{ 92 }^{ 235 }B\quad \) |
Question 6.
State the law of conservation of mass. Is this law applicable to the chemical reactions? Elaborate your answer with the help of an example.
Answer
(1) Law of conservation of mass states that mass can neither be created nor destroyed.
(2) Yes, this law is applicable to the chemical reactions. In all chemical reactions, there is an only exchange of reactants taking place when products are formed. There is no loss or gain of mass.
For example: In the following reaction, the total mass of the reactants is equal to the total mass of the products formed.
![]()
Question 7.
(1) What is the difference between molecular mass and molar mass?
(2) What relation exists between the atomic mass of an element and number of atoms? Explain with an example.
Answer
(1) The average mass of a molecule of a substance in the atomic mass unit is called molecular mass, whereas, the mass of 1 mole or 6.02 x 1023 molecules of the substance (in grams) is called its molar mass. Both these terms are numerically the same but differ in units. Molecular mass is expressed in the atomic mass unit (u), whereas, molar mass is expressed in grams.
(2) The mass of 6.02 x 1023 atoms, in grams is equal to its atomic mass. In other words, the atomic mass of every element contains 6.02 x 1023 atoms of that element.
For example, Atomic mass of magnesium is 24. So 24-gram magnesium has 6.02 x 1023 atoms of Mg, and a mass of 6.02 x 1023 atoms of magnesium is 24 gram.
Question 8.
What is Avogadro’s number and how is it related to the molecular mass of compounds? Explain with an example?
Answer
The number 6.02 x 1023 is called Avogadro’s number.
The mass, in grams of 6.02 x 1023 molecules of any substance is equal to its molar mass. In other words, molecules mass of any substance has 6.02 x 1023 molecules.
For example, molecular mass of water H2O = 2 + 16 = 18
.’. Molar mass of H2O = 18 g
.’. In 18 g of H2O molecules,
6.022 x 1023 molecules are there.
Question 9.
(a) What is the maximum number of electrons that can be present in the outermost shell of an atom?
(b) Draw a sketch of Bohr’s model of an atom with three shells.
Answer
(a) Eight.
Question 10.
(a) How are canal rays different from electrons in terms of charge and mass?
(b) What are canal rays? Who discovered them? What is the charge and mass of canal ray?
Answer
(a) Canal rays consist of positively charged particles, protons, having appreciable mass, while electrons are negatively charged with negligible mass.
(b) New radiations in a gas discharge tube which are positively charged are known as canal rays. They were discovered by E. Goldstein. Charge on canal rays is positive and its mass is one unit.
Question 11.
What information do you get from the diagrams given below about the atomic number, mass number, and valency of the atoms X, Y, and Z? Give your answer in a tabular form
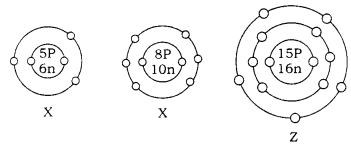
Answer
| Atom | Atomic Number |
Mass | Valency |
| X | 5 | 11 | 3 |
| Y | 8 | 18 | 2 |
| Z | 15 | 31 | 3, 5 |
Question 12.
State the major drawback in Rutherford’s model of an atom. Mention two features of Bohr’s Model which helped compensate this drawback.
Answer.
The major drawback of Rutherford’s model of an atom is that it does not explain the stability of an atom. Any particle in a circular orbit would undergo acceleration. During acceleration, charged particles would radiate energy. Thus, the revolving electron would lose energy and finally fall into the nucleus.
Two features of Bohr’s model which helped compensate this drawback are as follows:
- Only certain special orbits known as discrete orbits of electrons are allowed inside the atom.
- While revolving in these discrete orbits, the electrons do not radiate energy.
Atomic Structure Long Answer Type Questions
Question 1.
Find the % composition of the elements in the following compounds:
(1) Water
(2) Sodium Sulphate
Answer

Question 2.
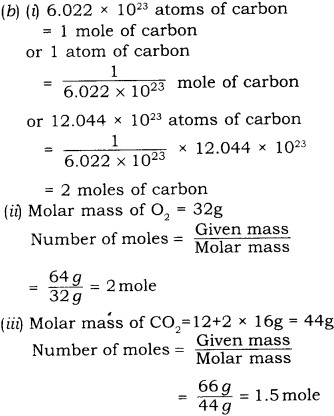
(1) Define one mole, illustrate its relationship with Avogadro constant,
(2) Calculate the number of moles in:
- 12.044 x 1023 atoms of carbon
- 64g of oxygen atoms
- 66g of carbon dioxide molecules.
(Atomic masses O = 16, C = 12)
Answer
(1) One mole of any species (atoms, molecules, ions or particles) is that quantity in number which has a mass equal to its atomic or molecular mass (in grams). The number of particles (atoms), molecules or ions) present in 1 mole of any substance is fixed, with a value of 6.022 x 1023. This number is called Avogadro constant or Avogadro number.
Question 3.
(a) Define:
(1) Molecular mass
(2) Avogadro constant.
(b) Calculate the number of molecules in 50g of CaCO3
(Atomic mass of Ca = 40 u, C = 12u and O=16u)
(c) If one mole of sodium atom weighs 23g, what is the mass (in g) of one atom of sodium?
Answer
(a)
(1) Molecular mass:
It is equal to the sum of the masses of atoms present in one molecule of a substance.
(2) Avogadro constant:
The number of atoms present in 12 gms of C-12-isotope.
\( \cfrac { Gram\quad atom\quad mass\quad of\quad carbon }{ Mass\quad of\quad one\quad carbon\quad atom } \)
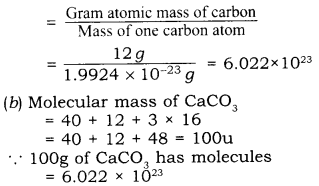
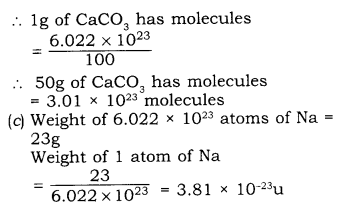
Question 4.
A number of electrons, protons, and neutrons in chemical species A, B, C, and D is given below.
| Element | Electrons | Protons | Neutrons |
| A | 2 | 3 | 4 |
| B | 10 | 9 | 8 |
| C | 8 | 8 | 8 |
| D | 8 | 8 | 10 |
Now answer the following questions:
1. What is the mass number of A and B?
2. What is the atomic number of B?
3. Which two elements represent a pair of isotopes and why?
4. What is the valency of element C? Also, justify your answers.
Answer
(1) Mass number of A = 3 + 4 = 7
Mass number of B = 9 + 8= 17
(2) The atomic number of B
= Number of protons = 9
(3) Elements C and D represent a pair of isotopes because their atomic numbers are the same, but mass numbers are different.
(4) Electronic configuration of C(8)=2, 6
So, its valency is 2.
Question 5.
Give reasons for the following:
(a) The nucleus of an atom is heavy.
(b) The nucleus of an atom is positively charged.
(c) An atom is electrically neutral.
Answer
(a) All the protons and neutrons are located in the nucleus. Proton and neutron each have a mass equal to 1 atomic mass unit. Electrons revolving outside the nucleus have negligible mass. Thus, the whole mass of the atom is concentrated in the nucleus. So the nucleus is heavy.
(b) All the positively charged particles, i.e. protons, are located in the nucleus. Negatively charged electrons are present outside the nucleus. Thus, the whole positive charge of the atom is concentrated in the nucleus. So the nucleus is positively charged.
(c) The number of protons and number of electrons in an atom is equal. So the two charges neutralize each other and the atom, on the whole, is electrically neutral.
We hope the given RBSE Solutions for Class 9 Science Chapter 3 Atomic Structure will help you. If you have any query regarding Rajasthan Board RBSE Class 9 Science Chapter 3 Atomic Structure, drop a comment below and we will get back to you at the earliest.