Rajasthan Board RBSE Class 12 Biology Notes Chapter 16 Plant Tissue Culture
Introduction
After discovery of cell by Robert Hooke in 1665, Metthias Jacob Schleiden and Theodor Schwann proposed cell theory in 1839. According to cell theory cell is structural and functional unit of life of any organism. In 1855, Rudolf virchow added in cell theory that new cells are formed from pre-existing cells (Omnis cellulae cellula).Confirmation of this fact by Rudolf Virchow cell theory proved as a milestone in the field of life sciences.
Modern Cell theory
- Cell is structural, functional and fundamental unit of life.
- New cells arise from pre-existing cells.
- All types of metabolic reactions take place in cell.
- Genetic information is transferred from one generation Introduction to next through cells.
According to cell theory each and every cell of any plant posses all characteristics through which whole plant can be regenerated. Capacity of producing complete plant from any cell is called “Totipotency” The term totipotency was first of all used by Morgan (1909). Due to this totipotency property, whole plant can be regenerated from a plant cell. This was experimentally proved by Haberlandt (1902).
![]()
Most of the animals lack the property of totipotency. Culture of plant protoplast, cell, tissue, organ or complete system on chemically known medium under sterile and controlled conditions is called Tissue culture. Plant tissue culture was first of all conducted by German scientist, Gottlieb Haberlandt (1902). Contribution of various scientists in the field of plant tissue culture has been shown in Table 16.1
Contribution of Various Scientists in field of Plant Tissue Culture
| Year | Name of Scientist | Discovery |
| 1902 | Gottlieb Haberlandt | First attempt to culture plant cell on artificial culture medium. |
| 1922 | W.J. Robbins and W.Kotte | Culture of Plant root and Shoot tip (Organ culture) |
| 1926 | F.W. Went | Discovered growth hormone and auxin (Indole acetic acid) |
| 1939 | R.J. Gautheret P.R. White P. Nobecourt | Established culture which can grow for long duration. |
| 1941 | Van Overbeek | First of all used coconut milk in culture medium for cell division. |
| 1946 | E. Ball | Developed clone of whole plant from culture of apical meristem. |
| 1954 | W.H. Murr | Developed the technique of culture of isolated single cell. |
| 1955 | F. Skoog and Miller | Established the role of Kinetin hormone in tissue culture technique for cell division. |
| 1959 | J. Rennert | Development of embryo from carrot root culture. |
| 1959 | R.J. Gautheret | Publication of Book “Handbook of Plant Tissue Culture”. |
| 1960 | E.C. Cocking | Separation or Isolation of protoplast by enzymatic digestion of Plant Cell wall. |
| 1962 | T. Murashige and F. Skoog | Developed most widely used culture medium (MS medium) in Plant Tissue Culture. |
| 1964 | S. Guha, Mukherjee and S.C. Maheshwari | Developed first haploid plant by culture of pollen grain of Datura |
| 1970 | J.B. Power | First of all demonstrated Protoplast fusion. |
| 1978 | G. Melchers et.al | Developed somatic hybrid Pom ato by fusion of protoplast of potato and tomato. |
| 1983 | M.D. Chilton | Culture of Transgenic plants of Tobacco. |
Some Important Terminology used in Tissue Culture Techniques
1. Culture Medium : The nutrient medium chemical composition of which is precisely known and which is used for Tissue culture and multiplication is called culture medium.
2. Explant : The part of the plant used for raising tissue culture. Example meristematic tissue, root, stem, leaves, flower and floral parts etc.
3. Callus : Unorganised mass of c’ells developed from newly cultured meristematic cells. These are generally paren- chymatous in nature.
4. Protoplast: Protoplasts are plant cells without cell wall.
5. Sterilization : The process of irradication of microbes or process of killing microbes, so as to make the object comptetely free from these.
![]()
6. Surface Sterilization : Removal of microbes from surface of explant by use of disinfectants, (e.g. Sodium hypochlorate) and then washing the explants with sterile distilled water under controlled conditions.
7. Inoculation : Transfer of surface sterilized explants on the suitable nutrient medium.
8. In vitro : Culturing in test tube, flask, bottle of glass or plastic vessels.
9. Clone : Population obtained from mother plant by vegetative propagation. All members of the clone are exactly similar to their parents genetically.
10. Somatic Embryo : The totipotent cells may undergo embryogenic pathway to form somatic embryos, which can be grown to regeneate into complete plant. They are generally larger in size than zygotic embryos.
11. Aritifical / Synthetic seed : Artificial seeds are produced by encapsulating the somatic embryos in a protective coating i.e. calcium alginate beads or by desiccating the somatic embryos with or without coating.
12. Embryoids : Somatic embryogenesis is an artificial process in which plant or embryo is derived from a single somatic cell or group of somatic cells. Somatic embryos are formed from plant cells that are not normally involved in the development of embryos i.e. ordinary plant tissue.
13. Micropropagation : Tissue culture method of plant propagation.
Resources required for Plant Tissue Culture
1. Tissue culture laboratory should be equipped with all modem equipments related to tissue culture. Normally this laboratory should not be established near microbiology and entomology related laboratories and places where seeds or plant parts are stored.Resources required for Plant Tissue Culture
2. While building the tissue culture laboratory, special care should be taken regarding it’s design and the position of working areas. This laboratory should have the glassware washing room, culture medium preparation chamber, record room, sterilized transfer chamber and culture room. It is necessary to build green house for acclimatization and hardening of plantlets developed through tissue culture and a nursery for their storage.
![]()
3. Various facilities, instruments and apparatus required for an ideal plant tissue culture laboratory have been shown in Table 16.2.
| Work Place | Necessary Requirements and Apparatus |
| 1. Sterilization of Glassware | Adequate water supply, Dishwasher, oven, etc. |
| 2. Preparation of Culture Media | Gas connection, Hot plates, heating metals, Physical and digital balance, water distillation plant, glass and ‘ plastic vessels, micropipette, fume hood, chemicals and stock solution, Autoclave, Refrigerator, centrifuge,magnetic stirrer, pH meter, culture tray, culture trolley etc. |
| 3. Recording the work (Paper work) | Chair, Table, Register, Almirah etc. |
The in vitro culture of plant parts or cells requires a variety of nutrients and suitable physical conditions, unlike the intact plants which can synthesize their own food and many other essential compounds needed for their growth and development, using light, CO2, water and minerals. The composition of plant tissue culture medium can vary, depending upon the type of plant tissues or cells that are used for culture.
1. Artificial medium through which we culture plant cell, tissue, organ or complete organ system is called culture medium. Depending on the experimental requirements, the medium can be liquid or semi solid. For making semi solid medium, a gelling agent such as agar (a polysaccharide obtained grom a red algae, Gelidium amansii)is Depending on the experimental requirements various scientists worked for developing nutrient media. Some of the important media developed are White, 1953. B. Gamborg et.al. 1968 and M.S.- Murashige and Skoog, 1962 medium.
2. Among these M.S. Medium is commonly used medium for general experiment of Plant tissue culture. M.S. medium is utilised after autoclaving (sterilization) and cooling it.
Steps of Micropropagation
Following steps are used in micropropagation method from selection of explant till it is developed in to complete plant.
Zero Step : This step is divided into two parts :
1. Selection of Explant and Pre treatment : Selection of explant depends on experimental requirements and objective of experiment. For different objectives, selection of suitable explant is shown in table 16.3. Before surface sterilization, selected explants are washed under running water with the help of chemical Tween – 20; which cleans dust particles and microbes from its surface. This process is called Pretreatment of explants.
Explants as per different objectives
| S.No. | Objective | Explant |
| 1. | Cloning | Stem tip, axilliary buds. |
| 2. | Virus free plants | Apical or axilliary meristem. |
| 3. | Somatic cloning propagation |
Any vegetative part of plant except meristematic tissue |
| 4. | Haploid plant culture | Pollen grain, anther lobe, unfertilized egg cell. |
| 5. | Protoplast culture |
Generally leaves |
| 6. | Triploid plant production | Endosperm |
| 7. | Somatic Embryogenesis |
New ly formed plant parts. |
| 8. | Callus culture | Any plant part. |
(ii) Surface Sterilization of Explant: Various types of surface sterlization chemicals (disinfectants) are used such as Mercuric chloride (HgCl2), Ethanol, Silver nitrate (AgNO3), Bromine and Chlorine water. Selected explants are surface sterilized by appropriate disinfectants in Laminar- air flow, which results in complete sterilization (Removal of all microbes) of explant.
- First Step : Initiation of Culture : Surface sterilized explant is transferred on a suitable nutrient medium and then transferred to culture room. In cutture chamber either callus or organ formation starts.
- Second Step : Transfer of culture of first step on to a fresh medium for multiplication.
Regeneration of Complete Plant:
- Third Step : Shoot initiation: Multiple shoot formation from the cultured explant.
- Rooting of Shoots : Rooting of in vitro developed shoots on rooting medium.
- Fourth Step : Transplantation : Hardening and acclimatization of tissue culture raised plants as they are tender. Subsequent transplantation to the green house or field.
Types of Culture
- On the basis of experimental objectives, tissue culture is of following types :
Callus Culture
1. Callus refers to an unorganised mass of cells, which are generally parenchymatous in nature. A variety of plant parts can be used to show callosing response, but the response varies with the composition of culture media. Generally auxins are added to culture medium for callus induction , but the nature and quantity of auxin added depends on the nature and source of explant and its genotype besides other factors :
2. Callus culture can be maintained for prolonged period by repeated sub culture. Callus cultures are used for :
- Plant regeneration
- Preparation of the single cell suspensions and protoplasts.
- Genetic transformation studies.
![]()
3. Following are two regeneration pathways to form plants from callus.
(i) Organogenesis
(ii) Somatic Embryogenesis.
(i) Organogenesis : Formation of organs like shoot root, bud etc. from the undifferentiated mass of cells (callus) is called organogenesis from the cultured explants. Miller and Skoog experimentally proved that formation of shoot or root first on the cultured tissue depends on the relative concentration of auxin and cytokinin.
If auxins are high in the medium then it promotes rooting while if cytokinins are high shoot formation is promoted. Development of root from callus is called Rhizogenesis and development of shoot is called Besides concentration of growth hormone, chemical composition physical state of nutrient medium, and nature of explant also determines root or shoot formation.
.
(ii) Somatic Embryogenesis : The totipotent cells may undergo embryogenic pathway to form somatic embryos which can be grown to regenerate into complete plants. Generally somatic embryos resemble the zygotic embry os (seed embryos) except in their place of origin and larger size. Steward (1958) and Reinert (1959)first independently reported the somatic embryogenesis from carrot cultures.
Organ Culture
It deals with the culture of the isolated organs (like roots) under laboratory conditions (in vitro). Different names are given depending upon the organ utilized for the culture. For instance root culture, ovary culture etc.
Embryo Culture (Embryo Rescue)
It is very difficult to produce hybrids in case of interspecific and inter-generic crosses (crosses between distantly related plants), because abnormal development of the endosperm causes premature death of the hybrid embry o and leads to sterile seeds. The embryo from such sterile hybrid seeds can be excised at an appropriate time and cultured on a suitable nutrient medium to produce novel hybrids which is otherwise not possible. This is known as Embryo Rescue. Several useful hybrids were produced in a variety of crops using this technique.
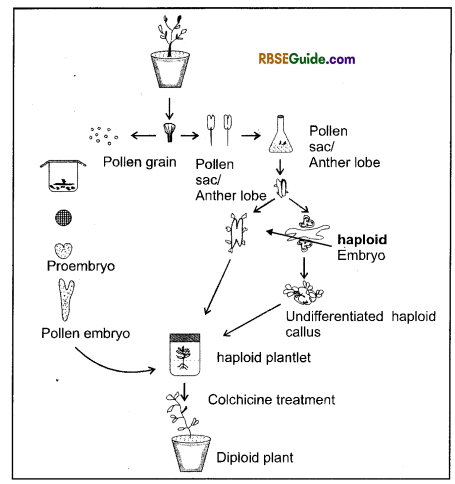
Anther and Pollen grain culture (Haploid Production)
Shimakure (1943) first of all carried out in vitro culture of pollen sac for studying meiotic cell division. Later on Indian scientists Shipra Guha Mukherjee and Satish Chandra Maheshwari (1964) were able to produce haploid plant from culture of pollen grain and pollen sac of Datura plant. Technique of producing haploids is used to obtain pure line to be used in plant breeding experimentation. Maximum number of haploids have been developed from plants of Solanaceae family.
The technique of haploid production through anther and pollen culture as well as ovary’ culture is of immense use in plant breeding to improve crop plants. It enables raising plants expressing traits that are otherwise recessive. The genetically homozygous diploid plants, which serve as parents in cross breeding can also be produced by diploidisation of haploid plants using colchicine chemical.
![]()
Cellsuspsension culture
Single cells can be isolated grom either callus or any other part of the plant (e.g. leaf) and cultured in liquid
medium. Both mechanical and enzymatic methods can be used for isolation of plant cells. Mechanical method involves grinding of the tissue in to a fine suspension in a buffere medium followed by filtration. Centrifugation is done to get rid of cell debris.
The enzymatic method is based on the usage of enzyme (pectinase/macerozyme), which dissolves the middle lamellae between the cells, i.e., the inter-cellular cementing layer, to release single cells. Once the cells have been isolated, they may be cultured by batch cultures or continuous culture. The cell suspension culture can be used for :
- Induction of Somatic Embryos/Shoots.
- In vitro mutagenesis and mutant selection.
- Genetic transformation.
- Production of secondary metabolites.
Protoplast Culture
Protoplasts are plant cells without cell wall and can be isolated from a variety’ of plant tissues (usually leaves, callus piece, single cells or pollen grains) by enzymatic method using cell wall digesting enzymes (cellulases, hemicellulases and pectinases). Protoplasts are usually cultured on a suspension culture in petri plates. As the protoplasts lack cell wall, they can be utilized for many purposes such as :
- Various biochemical and metabolic studies.
- Genetic transformation.
- Fusion of enucleated and nucleated protoplasts to produce cytoplasmic hybrids (cybrids).
- Fusion of two somatic cells to produce somatic hybrids.
Application and Achievements of Plant Tissue Culture
- Plant tissue culture has many application in field of Forestry’, Agriculture, Horticulture and Medicine production. Some of the important achievements are as follows : Application and Achievements of Plant Tissue Culture
Micropropagation
Vegetative propagation of plants is of considerable importance in agriculture, horticulture and forestry as it provides the multiplication of uniform material for crop planting (clones). Traditionally, it is done by using cuttings, budding, grafting, cor-ms, tubers and other vegetative propagules. The main problem with this method is that it is. labour-intensive, low producitivity and seasonal.
Tissue culture method of plant propagation, known as “micropropagation” can be used to overcome the problems mentioned above. This technique has been adopted for commercialisation of important plants (Nobel plants) such as different species of orchids Cattleya, Cymbidium, Dendrobium, Vanda and many ornamental plants, such as Gerbera, Guldaodi, Carnation, Begonia and certain other plants as banana, apple, pears, strawberry, cardamom etc.
Commercially important forestry and horticultural plants are fieing multiplied by micropropagation technique in various institutes and universities of India.
Regeneration of Virus free Plants
1. Most of the crop plants, particularly vegetatively propagated plants are systemically infected with viruses. If the stock of cultivar (vegetative propagules) is infected with virus, the entire clonal population raised with such stock will also be diseased and reduce the yield and quality signficantly.
Therefore, the production of virus free plants is important to increase yield and quality. Interestingly, the distribtuion of viruses in plants is uneven, and the apical or axillary meristems are generally free from viral particles. Small meristems (usually less than 1mm long) are collected from virus infected plants and then cultured.
2. Apical meristem culture technique has been used successfully to raise potato virus ‘S’ (PVS) free potato plants, potato virus ‘x’ (PVx) free potato plants and also in regeneration of virus free plants of sugarcane, banana and apple.
Production of Artificial or Encapsulated Seeds
1. The artificial seeds (also called as synthetic seeds, somatic seeds or encapsulated seeds) can be utilized for the rapid and mass propagation of elite plant species as well as hybrid varieties. Artificial seeds are produced either by encapsulating the somatic embryos in a protective coating, i..e calcium alginate beads or by desiccating the somatic embryos with or without coating. These artificial seeds germinate as a normal seed. This type of experiment was first of all done by Murashige (1977).
Embryo Rescue
- This technique is used to save embryo formed by
interspecific and inter-generic crosses. (Already discussed in detail under topic Embryo culture). - Production of Androgenic hapioids
- This technique of haploid production is of immense use in plant breeding to improve crop plants and in pure-line production (Already explained in detail under topic “Anther and Pollen grain culture).
Production of Triploid Plants
- In angiosperms the endosperm is a triploid tissue and an excellent material to produce triploid plants by culturing endosperm tissue. Triploid plants usually show seed sterility or seedlessness, which is desirable in crops like citrus, apple, and pear.
Note: Besides above applications, tissue culture technique is also used in following areas :
- Somatic hybridization
- In production of somatic clonal variations.
- Germplasm conservation
Gene Transfer in Plants
Transfer of genes and expression of these genes into plant cell is a modem application of plant tissue culture in the field of agriculture. For achieving genetic transformation in plants, the basic pre-requisite is the construction of a vector (genetic vehicle) which carries the gene of interest flanked by the necessary controlling sequences
i.e. promoter and terminator, and transfer the genes into the host plant. Transfer of genes and expression Of foreign genes into plant cells of important agricultural, forestry and medicinal plants will be studied under following heads as :
- Methods of gene transfer in plants
- Genetically modified plants.
![]()
Methods of gene transfer in Plants :Genes can be transferred in plants by several methods. The following two methods are more effective and in practice.
- Vector mediated or Indirect gene transfer.
- Direct gene transfer.
(a) Vector Mediated or Indirect Gene Transfer
In this procedure the DNA is transferred with the help of some vector. There, are three methods of this-
(i) Agrobacterium mediated Gene Transfer:
Among the various vectors used in plant transformation, the Ti-plasmid of Agrobacterium tumefacien’s has been used extensively. This bacterium contains large sized plasmid, known as Ti-plasmid (tumour-inducing plasmid) and portion of this plasmid referred as T-DNA (transferred DNA)
when is transferred to plant genome, in the infected cells, it causes plant tumours (crown galls). This means that A. tumefaciens has natural ability to transfer T-DNA of its plasmid into plant genome at the site of infection, and therefore this bacterium is known as ‘natural genetic engineer bf plants’. Because of this unique property.
Ti-plasmid can be used as vectors for inserting useful foreign genes into plant cells and tissues. The foreign genes (transgenes), i.e., the gene of interest (e.g. Bt gene for insect resistance) and plant selection marker gene, usually an antibiotic gene like nptll which confer resistance to kanamycin are cloned in place of the T-DNA region of Ti- plasmid.
There is a possibility o’f tumor formation in plants by Ti plasmid. Hence for developing transgenic plants the tumor inducing gene (T-DNA) is separated from the plasmid DNA of Agrobacterium and in place of this desired gene is incorporated. Now the desired gene containing Agrobacterium is cultured with the tissues of the plant in which the desired gene is to be transferred.
Normally the rings or discs of leaves of tomato, tobacco, Petunia, rose etc. are used for composite culture because the acetosyringone produced by the rings or discs of leaves activate the operons of Ti plasmid. As a result of activation of these operons the desired gene containing Ti plasmid enters in several cells and gets incorporated in the genome of the plant cells. After 2 to 3 days of composite culture, the transformed cells are cultured on suitable medium. This technique can be used for dicotyledon plants only.
Up to beginning of 20th century Agrobacterium tumefaciens and associated species were considered as caustive of plant diseases. But after knowing its ability to transfer foreign DNA, it is now used in Genetic engineering and due to extensive use in genetic engineering, it is known as Natural Genetic engineer.
Transformation of gene in plants can be detected on the basis of production of amino acids by the genes present in the plasmid of this bacterium. The genetically transformed cells produce opines. These opines are of different types which depend on the strain of the Agrobacterium. The strains of A. tumefaciens produce octapine end nopaline whereas strains of A. rhizogens produce agropine and manopine opines
In various research institutes, the scientists are using Ti plasmid as vector. By inserting desired and important genes in T DNA we can produce many important characteristics in plants such as Herbicide tolerance, Pathogen tolerance, Stress tolerance, increase in nutritive value (enrichment of rice with vitamin A, golden rice), improvement in nitrogen fixation.
Agrobacterium does not infect monocoto plants normally but in 1994, Japanese scientists were able to do transformation with the help of Ti plasmid in rice.
![]()
(ii) Virus Mediated Gene Transfer
Both DNA and RNA viruses act as ideal vector for transfer of desired genes. Two virus groups Caulimo viruses and Gemini viruses, which have DNA genome are most widely used DNA viruses for gene transfer. Retroviruses, Lentivirus and Adenovirus are also used widely in genetic engineering for gene transfer.
(iii) In-planta method :
This technique of gene transfer was developed by Failzadman and Markakes (1987). They kept Arabidopsis seed with genetically modified Agrobacterium and then raised the plants. Seeds obtained from these plants were germinated on antibiotic free medium and identified the transformed plants. Similarly the apical meristematic part of embryo of germinated seeds can also be infected with Agrobactermm to produce genetically modified plants. In this method of gene transfer, the genes are directly transferred into plant and for this reason this technique is called In planta technique.
(b) Direct gene transfer
In this method the DNA of desired gene(s) is transferred directly with some technique without involving any vector. Normally gene transfer by Agrobacterium is possible only in dicotyledons. In monocotyledons, which are chief cereals, gene transfer by Agroinfection is normally not possible. For improvement in these plants and to incorporate desired features, new techniques for gene transfer have been developed, in which biological vectors are not required. Hence the techniques in which biological vectors such as Agrobacterium or viruses are not required are called direct gene transfer.
Direct gene transfer in plants can be done by the following methods:
Chemical mediated gene transfer :
I Certain chemicals like polyethylene glycol (PEG), polyvinyl alcohol, calcium phosphate etc. induce DNA uptake into plant protoplasts Among chemical methods PEG is more commonly used chemical. In this method, first plasmid DNA is mixed with protoplasts and after some time 15-25% PEG is added. This amount of PEG promotes DNA uptake in the protoplasts.
The transformed protoplasts are cultured on selected medium and with the help of marker genes, the transformed protoplasts are selected. Liposomes, diethyl amino ethyl (DEAE) and dextron- proteins are also used for gene transfer in plants and animals. PEG directed gene transfer does not cause any harm to protoplasts.
Physical methods of Gene Transfer :
Direct gene transfer in plants is done effectively by several physical methods also. Some main physical methods are as follows.
(i) Gene gun : Gene gun is also known as particle gun, shot gun or microprojectile etc. By this technique gene transfer is possible in the intact plant cells, (cells having intact cell wall). This technique of gene transfer was first used by Klein and Co-workers (1987) in onion cells for transferring DNA and viral RNA.
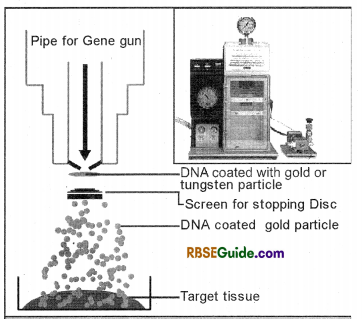
In this process the minute gold or tungsten particles of 1-3 mm diameter (microparticles) coated with desired DNA are shot (Bombarded) in the target cells with the help of microprojectile. These desired DNA coated gold or tungusten
particles penetrate the cell wall and enter the cell where desired DNA incorporates with the host cell DNA and forms transgenic DNA. By the use of this method gene transfer has been successfully achieved in wheat, rice, maize, tobacco and soyabean etc. This technique is being used now in all type of plants world wide.
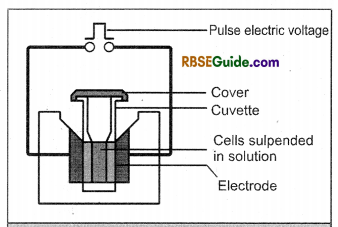
(ii) Electroporation : In this technique of gene transfer, the targeted protoplasts, plant cells or tissues are subjected to pulse of high voltage. As a result, minute temporary’ pores are formed in the plasmalemma (plasmamembrane). The desired DNA enter through these minute pores.
![]()
The target cells or the tissues are kept in solution containing the desired DNA and then subj ected to high voltage pulse. The DNA from the solution first enters into the cells and then gets incorporated in to the genome of the cells. This method is extensively used for transferring genes in the cells of monocotyledonous plants.
(iii) Liposome mediated gene transfer : In this method of gene transfer, spherical lipid molecules filled with desired DNA and water are used for transferring genes. These DNA containing lipid capsules first stick to the plasmalemma (plasma membrane) and then fuse with it. The DNA present in these first enter into the cell and then enter the nucleus where it gets incorporated in the genome of the host cell.
The liposome directed gene transferring technique also called as lipofection technique is highly effective technique for transferring genes in bacteria, animal & plant cells
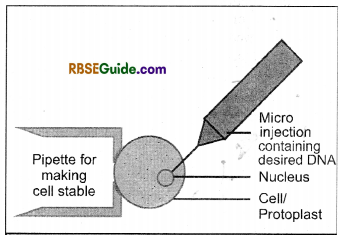
(iv) Microinjection : In this method genes are injected in plant protoplasts or cells with the help of glass needle of 0.5-1.0 mm diameter or micropipete directly in the cytoplasm or nucleus of the protoplasts or cells of plants. This is the suitable method of transferring genes in the isolated protoplasts.
Note : Besides the above methods of gene transfer other methods are also employed for transferring genes. These are Ladder dependent gene transfer and Silicon carbide fibre dependent gene transfer.
Selection of Genetically transformed Cells
1. Selection of genetically transformed cells is an important step in the field or genetic engineering. During transfer of desired gene, the marker gene (such as antibiotic resistant gene is also introduced in the genome of the host cell along with desired gene. This is done to select the transformed cells.
The transformed cells are then cultued on the medium containing suitable antibiotic and the transformed cells are separated. In this case, the transformed cells containing antibiotic resistant gene will grow’ in the culture and the untransformed cells fail to grow as these do not have the antibiotic resistant gene.
Genetically Modified Plants Or Transgenic Plants
Transgenic plants are genetically engineered plants’. These are developed by introducing new qualities through recombinant DNA techniqe during plant breeding programme. These new qualities pass in the progeny generation after generation. These genetically engineered plants or oranisms are called as genetically modified organisms (GMO). The products obtained from such plants are reffered as genetically modified food (GM food).
During last few decades many transgenic plants both among dicotyledons and monocotyledons, have been developed through genetic engineering and these are being tested in the crop fields.
Transgenic plants of desired species can be developed by the following steps-
- Identification and isolation of the gene of desired features.
- Formation of recombinant plasmid and transformation of Agrobacterium.
- Transfer of transformed Agrobacterium in the cells of target plant.
- Selection of transformed plant cells, their culture and regeneration.
- Expression of the desired gene in the developed transgenic plants.
Important Achievements in the field of Agricultural and Medical Science relating to Transgenic Plants
Pest Resistance
1. Bacillus thuringiensis bacterium commonly known as Bt (in short) is a soil inhabitant bacterium. This was first identified in 1911 and it was discovered that this was instrumental in causing death of larvae of four species of Boll worms. This bacterium was resistered as biopesticide in U.S.A. in 1961.
A gene called as cry gene is found in this bacterium. This gene (cry gene) codes for a protein which kills the insect pests. This gene was separated from Bt bacterium and was incorporated in the genome of cotton plant. The cotton variety so developed is called Bt cotton or killer cotton. This cotton is resistant to the Boll worm.
2. Several important crop plants are attacked by a variety of insects and pests, and cause significant reduction in the yield and quality. Farmers use synthetic pesticides extensively which cause adverse effects on human health and environment.
The transgenic technology provides an alternative and innovative method to apply pest control management which is eco-friendly, effective, sustainable and beneficial in terms of yield. The first gene identified for genetic engineering of crop plants for pest resistance were cry genes (popularly known as Bt genes) from the bacterium Bacillus thuringiensis.
Herbicide Resistance
1. Most herbicides normally kill the plants by preventing the synthesis of essential amino acids. Glyphosate is an herbicide which inhibits the activity of 5-end pyruvic shikimate-3 phospho synthase enzyme (EPSPs). This enzyme is required in the synthesis of aromatic amino acids.
![]()
In several plants gene regulating increased synthesis of EPSPs enzyme has been incorporated to reduce the effect of Glyphosate. Such transgenic plants have been developed in tomato, Petunia etc which show tolerance against most extensively used herbicide glyphosate sold as Roundup.
Development of Male sterility
Male sterile plants are highly important for hybridization in plant breeding programme. Availability of male sterile plant prevents undesired pollination and saves the time, labour and expences involved in the process of emasculation. In Rapseed (Toriya) plant male sterile line has been developed by incorporating barnase gene derived from Bacillus amyloliquefaciens. This gene (barnase gene) degenerates the tapetal cells in the anther lobes resulting into male sterility.
Delayed Fruit Ripening
The gaseous hormone ethylene is involved in regulating fruit ripening. Therefore, fruit ripening can be slowed down by reducing or blocking ethylene production. In U.S.A. a variety of tomato has been developed by incorporating the feature of reducing the amount of enzyme, polygalacturonase. This enzyme degrades the cell wall and is responsible for ripening of fruits. This variety of tomato knowns as Flavr Savr is genetically engineered variety. The tomato fruits of this variety remain fresh and tasteful for longer period as compared to the normal variety. ‘
Transgenic plants
As Bioreactors-Genetically modified plants are used as bioreactors. We know plants are like chemical factories and are capable of synthesizing several types of chemical compounds by using sunlight as the source of energy.
By incorporating right (desired) genes plants can be made to serve as bioreactors to produce desired type of compounds such as amino acids, proteins, vitamins, pharmaceutical products, enzymes etc. for use in food and medicine industry. Thus transgenic plants are being used to produce various types of chemicals. Because of this the field of biotechnology is called molecular farming.
Some of the plants developed for molecular farming are-
- “Golden Rice” (Vitamin A enriched)
- Super Potato
- Improved seed protein quality.
- Edible vaccines.
- Important medicines.
- Biodegradable plastic.
- Genetically engineered metabolism.
Transgenic plants with colourful Flowers
- In Netherland floriculture and marketing of flowers is an important trade. The scientist in Netherland isolated a gene from maize and transferred it inihe genome of Petunia and developed Petunia plants with orange coloured flowers. Similarly with the help of genetic engineering, blue rose, blue carnation and blue Tulips have also been developed.
Adverse effect of Transgenic plants on Environment, Ecology and Human beings
Genetically modified plants or Transgenic plants and the products produced by these have following safety concerns.
- The transferred genes of transgenic plants may be transferred to the crops of similar species and in related wild plants in nature.
- Apprehension of development of epidemic in crops in highly resistant varieties.
- The food products and other produce .of GM plants may cause allergy in the user.
- Apprehension of transfer of genes of animals, microbes and viruses in other important plants.
- Danger of destruction of natural biodiversity and begining of natural selection of new varieties.
- Impact on agricultural practice and imbalance in ecosystem.
Future Prospects of Transgenic plants
Despite of the above apprehensions and danger, the need and importance of transgenic plants can not be overlooked. In order to fulfil the requirements of the ever increasing population, scientists are engaged in developing transgenic plants by genetic engineering for the benefit of human culture and society. Some of the important prospects are as follows-
(i) Transfer of nitrogen fixing gene {nifgene) in cereal plants Future Prospects of Transgenic plants such wheat, rice etc for fixation of atmospheric nitrogen.
![]()
(ii) Recombination of nuclear and chloroplast genes from best sources so as to induce increase in photosynthesis and conversion of C3 plants in to more efficient C4 plants.
(iii) To develop such eco-friendly plants, which can degrade (decompose) the soil, water and atmospheric pollutants.
(iv) To incorporate changes in the vegetable and crop plants so that their growth and ripening becomes independent of climatic effects.