RBSE Solutions for Class 10 Science Chapter 5 Chemistry in Everyday Life are part of RBSE Solutions for Class 10 Science. Here we have given Rajasthan Board RBSE Class 10 Science Solutions Chapter 5 Chemistry in Everyday Life.
| Board | RBSE |
| Textbook | SIERT, Rajasthan |
| Class | Class 10 |
| Subject | Science |
| Chapter | Chapter 5 |
| Chapter Name | Chemistry in Everyday Life |
| Number of Questions Solved | 102 |
| Category | RBSE Solutions |
Rajasthan Board RBSE Class 10 Science Solutions Chapter 5 Chemistry in Everyday Life
Textbook Questions Solved
Multiple Choice Questions
RBSE Solutions For Class 10 Science Chapter 5 Question 1:
Aqueous solution (RBSESolutions.com) of a base
(a) Turns blue litmus into red
(b) Turns red litmus into blue
(c) Turns litmus solution colourless
(d) Has no effect on litmus solution
Answer:
(b) Turns red litmus into blue
RBSE Class 10 Science Chapter 5 Question 2:
What is the conductive property of aqueous solution of acid or base?
(a) Bad conductor
(b) Good conductor
(c) Semiconductor
(d) No effect
Answer:
(b) Good conductor
RBSE Class 10 Science Chapter 5 Question Answer Question 3:
pH shows negative logarithm of (RBSESolutions.com) concentration of which ions?
(a) [H2O]
(b) [OH–]
(c) [H+]
(d) [Na+]
Answer:
(c) [H+]
Class 10 RBSE Science Chapter 5 Question 4:
What will be the pH of any acidic solution?
(a) 7
(b) 14
(c) 11
(d) 4
Answer:
(d) 4
Chemistry In Everyday Life Class 10 Question 5:
The digestion in stomach takes (RBSESolutions.com) place in which type of medium?
(a) Acidic
(b) Alkaline
(c) Neutral
(d) Changing
Answer:
(a) Acidic
Ch 5 Science Class 10 RBSE Question 6:
Which of the following substances is used in making fire extinguisher?
(a) Sodium carbonate
(b) Sodium hydrogen carbonate
(c) Plaster of Paris
(d) Sodium chloride
Answer:
(b) Sodium hydrogen carbonate
RBSE Solution Class 10 Science Ch 5 Question 7:
Which of the following is the (RBSESolutions.com) formula of washing soda?
(a) NaHCO3
(b) NaCl
(c) CaSO4.1/2H2O
(d) Na2CO3.10H2O
Answer:
(d) Na2CO3.10H2O
RBSE Solution Class 10 Science Chapter 5 Question 8:
Which gas is released when bleaching powder is kept in open air?
(a) Hydrogen
(b) Oxygen
(c) Chlorine
(d) Carbon dioxide
Answer:
(c) Chlorine
RBSE Class 10 Science Chapter 5 In Hindi Question 9:
Soap works in which of the following?
(a) Soft water
(b) Hard water
(c) Both soft and hard water
(d) None of these
Answer:
(a) Soft water
Class 10 Science Chapter 5 RBSE Question 10:
Hydrocarbon tail is towards which (RBSESolutions.com) side in a micelle?
(a) Towards centre
(b) On surface
(c) Keeps changing
(d) In any direction
Answer:
(a) Towards centre
Chemistry In Everyday Life Class 10 RBSE Question 11:
Compounds which accept proton are
(a) Acid
(b) Salt
(c) None of these
(d) Base
Answer:
(d) Base
Chemistry in Everyday Life Very Short Answer Type Questions
Chapter 5 Class 10 Science RBSE Question 12:
Which acid is present in sting of red ant?
Answer:
Formic acid
RBSE Class 10 Science Chapter 5 Pdf Question 13:
Compounds which donate proton are (RBSESolutions.com) known by which name?
Answer:
Acid
Class 10 Science Chapter 5 Pdf Question 14:
What is neutralization?
Answer:
The reaction between acid and base produces salt and water. This reaction is called neutralization.
Class 10 Science Ch 5 Solutions Question 15:
Which process is used for killing germs in drinking water?
Answer:
Chlorination
Ncert Solutions For Class 10 Science Ch 5 Question 16:
How does a metal oxide react with acid? Write the chemical equation for this.
Answer:
Metal oxide is basic in nature thus, acid reacts with metal oxide as a neutralization reaction.
Metal oxide + Acid → Salt + water
e.g., CuO + 2HCl → CuCl2 + H2O
Acid Base And Salt Class 10 In Hindi Question 17:
What is the meaning ‘p’ and ‘H’ in pH?
Answer:
Here, ‘p’ means Potenz and ‘H’ means hydrogen.
Chemcistry Class 10 Solutions Question 18:
What will you take to get relief (RBSESolutions.com) from hyperacidity?
Answer:
Milk of magnesia
Ch 5 Science Class 10 Question 19:
Write the names of any two salts of sodium.
Answer:
Sodium chloride and sodium carbonate
Class 10 Chemistry All Chapter Name Question 20:
Define a base as per Lewis concept.
Answer:
Bases are substances which donate electron pair.
Class 10 Science Chapter 5 Question 21:
What is saponification?
Answer:
Fatty acid is heated with aqueous solution of sodium hydroxide or potassium hydroxide to obtain soap. This reaction is called saponification.
New Science In Everyday Life Class 7 Solutions Question 22:
What is the speciality of (RBSESolutions.com) detergent?
Answer:
Detergent is able to show cleansing action even in hard water.
Class 10 Science Chapter 5 Question Answer Question 23:
Which compound is used for casting plaster on a broken bone?
Answer:
Plaster of Paris
Class 10th Chemistry Chapter 5 Question 24:
The concentration of hydrogen ion in a solution is 1 x 10-4 gm mole L-1. Find the pH of this solution. Is it an acidic or a basic solution?
Answer:
pH = -log [1 x 10-4]
pH = 4 log10
H = 4
The solution is acidic because pH value is less than 7.
Chemistry in Everyday Life Short Answer Type Questions
RBSE Solution Class 10 Science Question 25:
Write the name and uses of two strong (RBSESolutions.com) acids and two strong bases.
Answer:
Strong Acid: Hydrochloric acid is used as bathroom cleaner and sulphuric acid is used as electrolyte in battery
Strong Base: Sodium hydroxide is used for making soap and potassium hydroxide is used as cleansing agent.
Class 10 Chapter 5 Science Question 26:
What is the difference between soap and detergent?
Answer:
| Soap | Detergent |
| Soaps are sodium or potassium salt of long chain fatty acids. | Detergents are alkyl benzene sulphonates. |
| They work in soft water but don’t work in hard water. | They even work in hard water. |
Importance Of Ph In Everyday Life Class 10 Question 27:
Define acids and bases as per Arrhenius concept.
Answer:
(a) Arrhenius definition for
- Acid: Acids are those substances which release H+ ions when dissolved in water.
- Base: Bases are those substances which release OH– ions when dissolved in water.
Chemistry In Everyday Life Class 10 Question 28:
What is pH? Explain pH range of acidic (RBSESolutions.com) and basic solutions.
Answer:
The negative logarithm of hydrogen ion concentration in a solution is calledpH of that solution. Let us assume that concentration of hydrogen ion in a solution is 1 x 10-4 gm mole L-1 .
The pH of this solution can be calculated as follows:
pH = -log [1 x 10-4]
pH = 41og1010
pH = 4
pH range is between 0 to 14. The pH of a neutral solution is 7. All basic solutions have pH more than 7. All acidic solutions have pH less than 7.
Chapter 5 Class 10 Science Question 29:
What is water of crystallization? Explain with (RBSESolutions.com) suitable example.
Answer:
(a) Arrhenius definition for
- Acid: Acids are those substances which release H+ ions when dissolved in water.
- Base: Bases are those substances which release OH” ions when dissolved in water.
Preparation Of Plaster Of Paris Class 10 Question 30:
What happens when:
(i) Curd or a sour substance is kept in metallic container.
(ii) Teeth are not cleaned after dinner.
Answer:
(i) Curd or sour substance has acid in it. The acid reacts with metal to produce hydrogen gas.
Hence, any sour substance gets spoiled and damages the metallic container if it is kept in a metallic container.
(ii) Bacteria feed on food particles stuck between teeth. While doing so, they produce acid which damages the teeth. So, if teeth are not cleaned after dinner, it poses a risk of tooth decay.
Plaster Of Paris Formula Class 10 Question 31:
A substance A reacts with sulphuric acid and produces a gas B with effervescence. Gas B bums with a pop sound. Name A and B and write the equation for this reaction.
Answer:
Substance A is a metal (example: Zinc) and gas B is hydrogen gas. This reaction can be shown by following equation:
Zn + H2SO4 → ZnSO4+ H2
Chemistry in Everyday Life Long Answer Type Questions
Chemistry In Everyday Life Class 12 Pdf Question 32:
Explain acid and base on the basis of Bronsted (RBSESolutions.com) Lowry and Lewis concepts.
Answer:
Bronsted Lowry Concept of Acid and Base: As per this concept, acids are proton donor while bases are proton acceptor.
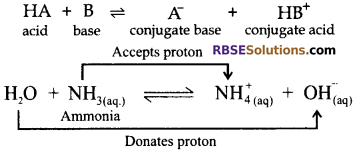
In this example, water is proton donor and hence it is acting like an acid. Ammonia; being proton acceptor, is a base.
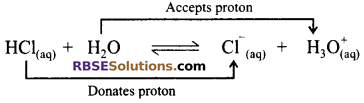
In this example, water is proton acceptor and hence acting like a base. Hydrochloric acid being proton donor, is an acid.
Lewis Concept of Acid and Base:
Acids are substances which accept electron pair, while bases are substances which donate electron pair.
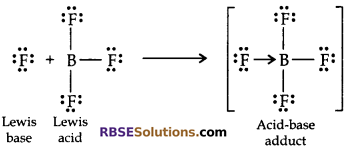
Life Science Class 10 Question 33:
What is the significance of pH in (RBSESolutions.com) everyday life?Answer:
Importance of pH in Everyday Life
- Our body works within pH range of 7.0 to 7.8.
- Acid rain, (pH – 5.6) affects aquatic animals.
- Plants need specific range of pH of soil.
- Acids produced by stomach helps in digestion. If too much acid is formed, people take antacids e.g., milk of magnesia.
- Tooth decay starts when pH in mouth is lower than 5.5. Bacteria present in mouth produce acids by degradation of sugar and food particles remaining in the mouth. Rinsing mouth after eating and using toothpastes which are basic are usually practised for prevention of tooth decay.
- Bee-stings produce acids which causes pain and irritation. Use of baking soda on the sting area gives relief. Stinging hair of nettle leaves inject methanoic acid causing burning pain.
Class 10th Chapter 5 Science Question 34:
Write the name, method of production and (RBSESolutions.com) uses of following:
(i) NaOH
(ii) NaHCO3
(iii) Na2CO3.10H2O3
(iv) CaOCl2
(v) CaSO4 .1/2H2O
Answer:
(i) Name: Sodium hydroxide
Method of Production: This is made by chlor-alkali process. Aqueous solution of sodium chloride (brine) is electrolysed to obtain NaOH.
2 NaCl + 2 H2O \(\underrightarrow { electrolysis }\) NaOH + Cl2 + H2
Uses: It is used for making paper, soap, detergents and artificial fibres.
(ii) Name: Sodium bicarbonate or baking soda Method of Production: It is made by reaction between sodium chloride, ammonia, carbon dioxide and water.
NaCl + H2O + CO2 + NH3 → NH4Cl + NaHCO3
Uses: It is used as antacid, in soda-acid fire extinguisher and for making baking powder.
(iii) Name: Washing soda
Method of Production: When baking soda is heated, we get sodium carbonate. It is then crystallized by adding water.
2 NaHCO3 → Na2CO3+ H2O+ CO2
Na2CO3 + 10 H2O → Na2CO3.10H2O
Uses: It is used in making glass, paper, soap, borax. It is used as cleansing agent. It is used for removing permanent hardness from water.
(iv) Name: Bleaching powder
Method of Production: It is made by action of chlorine on dry slaked lime.
Ca(OH)2 + Cl2 → CaOCl2 + H2O
Uses: It is used for bleaching cotton, linen, wood pulp, etc. It is used as oxidizing agent in chemical industry. It is used for disinfecting drinking water.
(v) Name: Plaster of Paris
Method of Production: This is made by heating gypsum.
2CaSO4.2H2O 2CaSO4. \(\frac { 1 }{ 2 }\) H2O+ 3H2O
Uses: Used for making plaster cast over fractured bones. Used for making artifacts, false ceilings, etc.
Science Class 10 Ch 5 Question 35:
Explain the formation and (RBSESolutions.com) working of micelle.
Answer:
Soap molecule has two ends, the charged end that gets attracted towards water is called hydrophilic and the long carbon chain that repels water is called hydrophobic end. When soap is dissolved in water, the carbon chain i.e., hydrophobic end gets attracted towards the oil, dirt and grease. The hydrophilic end which is attracted by water molecules points outwards thus, the micelle formation takes place.
The tail entangles dirt, oil or grease, if required the agitation is done. Lot of rinsing is done with water so that water molecules attract charged (Na+) end and carries the soap molecules with dirt attached to it and clean the clothes, utensils, etc.
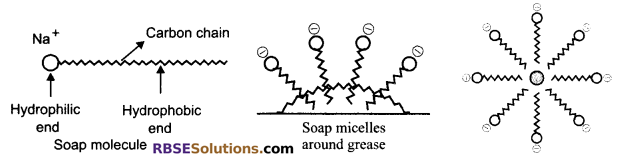
Chemistry in Everyday Life Additional Questions Solved
I. Multiple Choice Questions
Science Class 10 Chapter 5 Question 1:
A milkman added a small pinch of baking soda to (RBSESolutions.com) fresh milk which had pH close to 6. As a result, pH of the medium
(a) became close to 2
(b) became close to 4
(c) did not undergo any change
(d) became close to 8
Answer:
(d) became close to 8
Class 10 Science Chapter 5 Question Answer In Hindi Question 2:
In which of the following pairs, both are acidic salts?
(a) KCl, KNO3
(b) Na2SO4, K2SO4
(c) CH3COONa, K2CO3
(d) CuSO4, AgNO3
Answer:
(d) CuSO4, AgNO3
Ch 5 Science Class 10 Important Questions Question 3:
The compound used for neutralisation of (RBSESolutions.com) excess HCl in the stomach is
(a) NaHCO3
(b) Mg(OH)2
(c) Both
(d) None of these
Answer:
(c) Both
Class 10th Science Chapter 5 Question 4:
Which of the following is incorrectly matched?
(a) Tomato – tartaric acid
(b) Citrus – citric acid
(c) Ant sting – methanoic acid
(d) Curd – lactic acid
Answer:
(a) Tomato – tartaric acid
Chapter 5 Class 10 Science Ncert Solutions Question 5:
A solution reacts with crushed egg shells to give a (RBSESolutions.com) gas that turns lime water milky. The solution contains
(a) NaCl
(b) HCl
(c) LiCl
(d) KCl
Answer:
(b) HCl
Plaster Of Paris Ka Anusutra Question 6:
The aqueous solution of which of the . following salt will have OH– ions?
(a) NaCl
(b) Na2SO4
(c) CH3COONa
(d) None of these
Answer:
(c) CH3COONa
Water Of Crystallization Definition Class 10 Question 7:
Which of the following gives the correct increasing (RBSESolutions.com) order of acidic strength?
(a) Water < Acetic acid < Hydrochloric acid
(b) Water < Hydrochloric acid < Acetic Acid
(c) Acetic acid < Water < Hydrochloric acid
(d) Hydrochloric acid < Water < Acetic Acid
Answer:
(a) Water < Acetic acid < Hydrochloric acid
Question 8:
Which of the following phenomenon occur when a small amount of acid is added to water?
(i) Ionisation
(ii) Dilution
(iii) Neutralisation
(iv) Salt formation
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (i) and (iii)
(d) (ii) and (iv)
Answer:
(a) (i) and (ii)
Question 9:
Identify the correct representation of reaction (RBSESolutions.com) occurring during chior-alkali process.
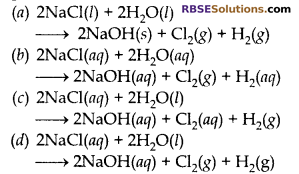
Answer:
![]()
Question 10:
The chemical formula of caustic potash is
(a) NaOH
(b) Ca(OH)2
(c) NH4OH
(d) KOH
Answer:
(d) KOH
Question 11:
Substances exposed to atmosphere at ordinary (RBSESolutions.com) temperature, lose their water of crystallisation are called as
(a) hygroscopt
(b) efflorescent
(c) deliquescent
(d) all of above
Answer:
(c) deliquescent
Question 12:
Which one of the following types of medicines is used for treating indigestion?
(a) Antibiotic
(b) Antacid
(c) Analgesic
(d) Antiseptic
Answer:
(b) Antacid
Question 13:
The composition of aqua regia is
(a) cone. H2SO4 and cone. HCl in ratio of 1 : 3
(b) cone. HNO3 and cone. HCl in ratio of 1 : 3
(c) cone. HNO3 and cone. HCl in ratio of 3 : 1
(d) cone. H2SO4 and cone. HNO3 is ratio of 3 : 1
Answer:
(b) cone. HNO3 and conc. HCl in ratio of 1 : 3
Question 14:
An element ‘X’ forms a solid oxide which (RBSESolutions.com) dissolves in water forming solution which turns blue litmus paper red, ‘X’ is
(a) Ca
(b) Cu
(c) Fe
(d) P
Answer:
(d) P
Question 15:
The formula of washing soda is
(a) NaHCO3
(b) Na2CO3. H2O
(c) Na2CO3
(d) Na2CO3.10H2O
Answer:
(d) Na2CO3.10H2O
Question 16:
The substance which on treating with chlorine, yields bleaching powder is
(a) quick lime
(b) limestone
(c) slaked lime
(d) gypsum
Answer:
(c) slaked lime
Question 17:
If tartaric acid is not added in baking powder, the (RBSESolutions.com) cake will taste bitter due to the presence of
(a) sodium hydrogen carbonate
(b) sodium carbonate
(c) carbon dioxide
(d) same unreacted tartaric
Answer:
(b) sodium carbonate
Question 18:
An aqueous solution has [H+ ] ion concentration = 1.0 x 10-7mol-1. Its pH value is
(a) +7
(b) -7
(c) 0.70
(d) 10-7
Answer:
(a) +7
Question 19:
Milk of magnesia is
(a) solid magnesium oxide
(b) solid magnesium hydroxide
(c) suspension of magnesium hydroxide
(d) insoluble magnesium carbonate
Answer:
(c) suspension of magnesium hydroxide
Question 20:
The pH of human blood varies between
(a) 4 to 5.5
(b) 7 to 7.8
(c) 5 to 6.2
(d) 9 to 10.2
Answer:
(b) 7 to 7.8
Question 21:
Which of the following statements is correct (RBSESolutions.com) about an aqueous solution of an acid and of a base?
(i) Higher the pH, strong the acid
(ii) Higher the pH, weaker the acid
(iii) Lower the pH, stronger the base
(iv) Lower the pH, weaker the base
(a) (i) and (iii)
(b) (i) and (iv)
(c) (ii) and (iii)
(d) (ii) and (iv)
Answer:
(d) (ii) and (iv)
Question 22:
Calcium phosphate is present in tooth enamel, its nature is
(a) basic
(b) amphoteric
(c) acidic
(d) neutral
Answer:
(a) basic
Question 23:
Which of the following salts does not contain (RBSESolutions.com) any water of crystallisation?
(a) Blue vitriol
(b) Washing soda
(c) Baking soda
(d) Gypsum
Answer:
(c) Baking soda
Question 24:
A sample of soil is mixed with water and allowed to settle. The clear suspended solution turns the pH paper yellowish- orange. Which of the following would change the colour of this pH paper to greenish-blue?
(a) Lemon juice
(b) An antacid
(c) Common salt
(d) Vinegar
Answer:
(b) An antacid
Question 25:
The pH of a solution of HCl is 4. This shows that the molarity of the solution is
(a) 4.0 M
(b) 0.4 M
(c) 0.0001 M
(d) 0.001 M
Answer:
(c) 0.0001 M
Question 26:
The difference of molecules of water in (RBSESolutions.com) gypsum and Plaster of Paris is
(a) 5/2
(b) 2
(c) 3/2
(d) 1/2
Answer:
(c) 3/2
Question 27:
Which of the following does not form an acidic salt?
(a) Phosphoric acid
(b) Carbonic acid
(c) Hydrochloric acid
(d) Sulphuric acid
Answer:
(b) Carbonic
Chemistry in Everyday Life Very Short Answer Type Questions
Question 1:
What is an acid?
Answer:
A substance which is sour in taste and which (RBSESolutions.com) turns blue litmus to red is called acid.
Question 2:
What is a base?
Answer:
A substance which is bitter in taste, soapy to touch and turns red litmus to blue is called base.
Question 3:
Which acid is secreted in stomach?
Answer:
Hydrochloric acid
Question 4:
Which acid is present in vinegar?
Answer:
Acetic acid
Question 5:
Which ions are released by aqueous (RBSESolutions.com) solution of acid?
Answer:
Hydrogen ion
Question 6:
Which ions are released by aqueous solution of base?
Answer:
Hydroxide ion
Question 7:
What is the pH of distilled water?
Answer:
7
Question 8:
What is a strong acid?
Answer:
An acid which produces (RBSESolutions.com) high amount of hydrogen ion in aqueous solution is called strong acid.
Question 9:
What is a salt?
Answer:
The product of neutralization reaction is called salt.
Chemistry in Everyday Life Short Answer Type Questions
Question 1:
A white powder which sets hard on adding water is also used in hospitals. Name this powder. How is it prepared? Write the chemical reaction involved in its preparation.
Answer:
The white powder is Plaster of Paris
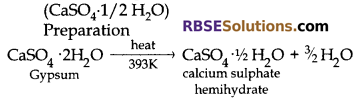
It is prepared by heating gypsum at 393 K.
Question 2:
Write balanced chemical equations for (RBSESolutions.com) the following:
(a) Calcium carbonate reacts with hydrochloric acid
(b) Dilute sulphuric acid reacts with zinc granules
(c) Calcium oxychloride reacts with hydrochloric acid.
Answer:

Question 3:
Name the ions present in the following salts. Name the acid and base from which they (RBSESolutions.com) can be obtained. magnesium sulphate, sodium carbonate, potassium chloride.
Answer:
The ions present in the compounds are:
| Salt | Ions | Acid required | Base required |
| (1) Magnesium sulphate | Mg2+ + SO2-4 | H2SO4 | Mg (OH)2 |
| (2) Sodium carbonate | Na+ + CO2+3 | H2CO3 | NaOH |
| (3) Potassium chloride | k+ + Cl– | HCl | KOH |
Question 4:
Give three ways in (RBSESolutions.com) which salts can be prepared.
Answer:
Salts can be obtained in the following ways:
(i) Acid reacts with base to give salt and water.
HCl + KOH → KCl + H2O
acid base salt
(ii) Metals react with acids to form salt and give hydrogen gas.
Mg + 2HCl → MgCl2 + H2
(iii) Metallic oxide reacts with acid to form salt.
CuO + 2HCl → CuCl2 + H2O
Question 5:
Give two examples for each of the following acids salts – chloride salts, nitrate salts (RBSESolutions.com) and sulphate salts.
Answer:
Chloride salts → Magnesium chloride, Calcium chloride
Nitrate salts → Ammonium nitrate, Aluminium nitrate
Sulphate salts → Calcium sulphate, Magnesium sulphate
Question 6:
Name the acid present in the following: Vinegar, Lemon, Tomato, Tamarind, Orange, Curd.
Answer:
| Source | Acid |
| Vinegar | Acetic acid |
| Lemon | Citric acid |
| Tomato | Oxalic acid |
| Tamarind | Tartaric acid |
| Orange | Citric acid |
| Curd | Lactic acid |
Question 7:
Name the properties (RBSESolutions.com) responsible for the following uses of baking powder:
(i) Baking industry
(ii) As an antacid
(iii) As soda-acid fire extinguisher
Answer:
Properties of baking powder Uses
(i) On heating releases CO2 gas Baking industry
(ii) Alkaline in nature, neutralises Antacid excess acid in stomach
(iii) When it reacts with Soda-acid fire
acid it releases CO2 gas extinguisher
which can extinguish fire.
Question 8:
Give the properties and uses of bleaching powder.
Answer:
Bleaching powder on reaction with dilute acid gives chlorine gas which (RBSESolutions.com) reacts with water to give nascent oxygen.
Uses
(i ) Bleaching Used in bleaching cotton, wood pulp, clothes.
(ii) Oxidising agent Used in chemical industries.
(iii) Disinfectant To kill germs in drinking water.
Question 9:
Acid when reacts with metal release hydrogen gas but there is one acid which when reacts with metal does not release hydrogen except for two metals. Prove this statement.
Answer:
Acid + Metal → Salt + Hydrogen
e.g., 2HCl + 2Na → 2NaCl + H2
H2SO4 + 2Na → Na2SO4 + H2
HNO3 + Na → Nohydrogengas Nitric acid does not release hydrogen gas when it reacted with metals.
This is because nitric acid is strong oxidising agent.
Nitric acid reacts only (RBSESolutions.com) with magnesium and manganese to evolve hydrogen gas
Mg + 2HNO3 → Mg(NO3)2 + H2 ↑
Mn + 2HNO3 → Mn(NO3)2 + H2 ↑
Question 10:
Give six uses of acids.
Answer:
Uses of acids are:
(i) In storage batteries (H2 SO4 )
(ii) As food preservative (acetic acid)
(iii) In the preparation of baking powder (tartaric acid)
(iv) In manufacturing of fertilizers (nitric acid)
(v) In making PVC (Poly vinyl chloride) (Hydrochloric acid)
(vi) As bathroom cleaner (hydrochloric acid)
Question 11:
Give six uses of bases.
Answer:
Uses of bases are:
(i) In making soap (NaOH)
(ii) As an antacid (Mg(OH)2)
(iii) In making bleaching powder (Ca(OH)2 )
(iv) In removing acidity of soils (Ca(OH)2 )
(u) In white washing (Ca(OH)2 )
(vi) In making fertilizers (NH4 OH)
Question 12:
Give six uses of salts.
Answer:
Uses of salts are:
(i) Adds taste to food (NaCl)
(ii) In making a freezing mixture (NaCl)
(iii) As washing soda for clothes (Na2CO3)
(iv) As baking soda (NaHCO3)
(v) To purify water (alum, CaOCl2)
(vi) In making of soaps (NaCl)
Question 13:
Four samples A, B, C and D were given to test their nature. A (RBSESolutions.com) student found the change in pH paper as follows:
A → green colour
C → blue colour
B → orange colour
D → pink colour
Find the nature of given sample.
Answer:
| Sample | Colour change | pH | Nature |
| A | Green | 7 | Neutral |
| B | Orange | 4 | Weak Acid |
| C | Blue | 9 | Base |
| D | Pink | 2 | Strong Acid |
Question 14:
How will you test for the gas which is liberated when hydrochloric acid reacts with an active metal?
Answer:
An active metal reacts with hydrochloric acid to give hydrogen gas. When a burning splinter is brought close to the gas evolved, pop sound is produced and the gas continues to burn.
Question 15:
What is ‘Baking Powder’? How does it make the cake (RBSESolutions.com) soft and spongy?
Answer:
Baking powder is the mixture of baking soda and tartaric acid.
When baking powder is heated it releases CO2 gas due to which the cake rises and become soft and spongy.
Question 16:
Fresh milk has a pH of 6. When it changes into curd (yogurt) will its pH value increase or decrease? Why?
Answer:
When fresh milk turns into curd, its pH value will decrease. This is because lactic acid is produced when milk changes into curd.
Question 17:
A compound which is prepared (RBSESolutions.com) from gypsum has the property of hardening when mixed with a proper quantity of water. Identify the compound. Write the chemical equation for its preparation. For what purpose is it used in hospitals?
Answer:
The compound is calcium sulphate hemihydrate, Plaster of Paris
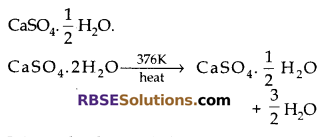
It is used in hospitals for plaster cast.
Question 18:
(a) Give Arrhenius definition of an acid and a base.
(b) Choose strong acid and strong base from the following:
CH3COOH, NH4OH, KOH, HCl
Answer:
(a) Arrhenius definition for
Acid: Acids are those substances which release H+ ions when dissolved in water.
Base: Bases are those substances which release OH” ions when dissolved in water.
(b) The strong acid is HC1 and strong base is KOH.
Question 19:
(a) Write the formula and chemical name of bleaching (RBSESolutions.com) powder.
(b) Write chemical equation to represent the action of atmospheric CO2 gas on bleaching powder when left exposed in open.
(c) State for what purpose is bleaching powder used in water treatment plants.
Answer:
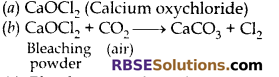
(c) Bleaching powder is disinfectant, it can kill germs and hence, it is used in water treatment plant.
Question 20:
Name the gas evolved when dilute HCl reacts with sodium hydrogen carbonate. How is it recognised?
Answer:
The gas evolved is CO2.
It can be recognised by passing it through lime water (freshly prepared) the lime water turns milky.
![]()
Question 21:
How is the pH of (RBSESolutions.com) a solution of an acid influenced when it is diluted?
Answer:
Acid when dissolved in water the H+ concentration in moles per litre decreases and therefore pH will increase, as the solution becomes less acidic on dilution.
a test tube containing dry slaked lime Ca(OH), bleaching powder is produced.
Question 22:
How does the pH of the solution change when a solution of base is diluted?
Answer:
Bases on dilution with water become less basic in nature and their pH decreases,
(e.g. pH of strong base wrould be 14, on diluting its pH becomes below 14)
Question 23:
Arrange the following in increasing order of their pH values:
NaOH solution, blood, lemon juice.
Answer:
Lemon juice < Blood < NaOH solution.
Question 24:
A compound ‘X’ of sodium is commonly used in (RBSESolutions.com) kitchen for making crispy pakoras. It is also used for curing acidity in the stomach. Identify ‘X’. What is its chemical formula? State the reaction which takes place when it is heated during cooking.
Answer:
‘X’ is sodium hydrogen carbonate (baking soda)
Chemical formula → NaHCO3
During cooking → It decomposes to
sodium carbonate, carbon dioxide and water
2NaHCO3 Na2CO3, + C02 + HzO
Question 25:
Why does tooth decay start when the pH of mouth is lower than 5.5?
Answer:
When the pH in mouth is lower than 5.5, it means acids are formed due to food decay by bacteria in our mouth. The acid released reacts with enamel, which is made up of calcium phosphate and tooth decay starts.
Question 26:
Which of these has a higher (RBSESolutions.com) concentration of H+ ions – 1 M HCl or 1M CH3COOH?
Answer:
1M HC1 has higher concentration of H+ ions because HCl– is a strong acid and ionise completely to give H+ and Cl– ions but CH3COOH is a weak acid which does not undergo complete ionisation.
Class 10 Science Rajasthan Board Chapter 5 Chemistry in Everyday Life Long Answer Type Questions
Question 1:
Design an activity to prove that acids show acidic behaviour only when dissolved in water.
Answer:
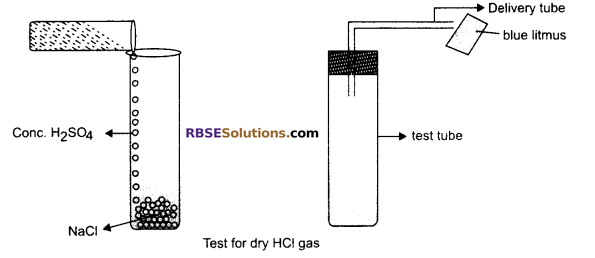
Take a clean test tube and add solid NaCl in it. Add some concentrated sulphuric acid in it, Fix the mouth of the test tube with cork and fix delivery tube in it. The reaction begins and gas comes out from the delivery tube, it is HC1 gas, Take a litmus paper (dry) and test the presence of gas, there will be no colour change, Now wet the litmus paper and bring it near the mouth of delivery tube. The blue litmus paper turns red. This activity proves that hydrogen ions in HC1 are produced in the presence of water.
Question 2:
What is bleaching powder? How is bleaching (RBSESolutions.com) powder produced? Give its chemical equation and write its three uses.
Answer:
Bleaching powder is calcium oxychioride CaOCl2.
Preparation: On passing chlorine gas through a test tube containing dry slaked lime Ca(OH)2 bleaching powder is produced.
Ca(OH)2 + Cl2 → CaOCl2 + H2O
Uses:
- It is used as bleaching agent in textile industry and paper factories.
- It is used as an oxidising agent in chemical industries.
- It is used as disinfectant for drinking water, to make it germ free.
Question 3:
What is baking soda and baking (RBSESolutions.com) powder chemically? What would happen if we add baking soda in making cakes instead of baking powder?
Answer:
Baking soda is NaHCO3 – Sodium hydrogen carbonate
Baking powder is NaHCO3 + Tartaric acid. If we add baking soda in making of cakes on heating it will produces sodium carbonate which will add bitter taste to the cake. To avoid this, tartaric acid is added to baking soda which produces sodium salt of acid and does not change the taste of cake.
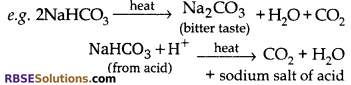
Question 4:
Write the chemical formula for washing soda. (RBSESolutions.com) How may it be obtained from baking soda? Name an industrial use of washing soda other than washing clothes.
Answer:
Washing soda → Na2CO3.10H2O. (sodium carbonate decahydrate)
Baking soda on heating produces sodium carbonate which is then recrystallised.
2NaHCO3 \(\underrightarrow { heat }\) Na2CO3 + CO2+ H2O
Na2CO3 + 10H2→ Na2CO3.10H2O
It is used in the manufacture of glass, paper.
Question 5:
(a) Why does an aqueous solution (RBSESolutions.com) of an acid conduct electricity?
(b) How does the concentration of hydronium ions (H3O+) change when a solution of an acid is diluted?
(c) Which has higher pH value, a concentrated or dilute solution of hydrochloric acid?
(d) What do you observe on adding dilute hydrochloric acid to
(i) Sodium carbonate placed in test tube.
(ii) Zinc metal in a test tube?
Answer:
(a) Aqueous solution of an acid releases ions so that it can conduct electricity.
(b) When solution of an acid is diluted, concentration of hydronium ions (H3O+) decreases.
(c) Dilute solution of HC1 will have higher pH than concentrated solution.
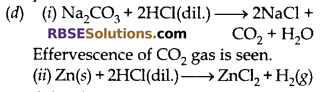
Odourless and colourless gas hydrogen is evolved (RBSESolutions.com) with bubbles on surface of zinc.
We hope the given RBSE Solutions for Class 10 Science Chapter 5 Chemistry in Everyday Life will help you. If you have any query regarding Rajasthan Board RBSE Class 10 Science Solutions Chapter 5 Chemistry in Everyday Life, drop a comment below and we will get back to you at the earliest.