Rajasthan Board RBSE Class 12 Chemistry Chapter 2 Solution
RBSE Class 12 Chemistry Chapter 2 Text Book Questions
RBSE Class 12 Chemistry Chapter 2 Multiple Choice Questions
RBSE Solutions For Class 12 Chemistry Chapter 2 Question 1.
4 g of NaOH is dissolved in 500 g of water. The concentration of solution will be
(a) 8 g/L
(b) 0.2.N.
(C) 0.2 m
(d) 0.2 M
RBSE Class 12 Chemistry Chapter 2 Question 2.
Which liquid pair represents positive deviation from Raoult’s law ?
(a) Water + HCl
(b) Water + HNO3
(c) Benzene + Methanol
(d) Acetone + Chloroform
RBSE Solutions For Class 12 Chemistry Question 3.
The molarity of pure water is-
(a) 55.5 M
(b) 100 M
(c) 18 M
(d) IM
RBSE Class 12 Chemistry Solutions Question 4.
Arrange the following 0.1 M solutions in increasing order of their boiling points
(a) NaCl
(b) MgCl2
(c) Urea
(d) AICI3
(a) (i) < (ii) < (iii) < (iv).
(b) (ii) < (i) < (iii) < (iv)
(c) (iii) < (i) < (ii) < (iv)
(d) (iv) < (iii) < (ii) < (i)
RBSE Chemistry Solution Class 12 Question 5.
This is a property of an ideal solution
(a) It obeys Raoult’s law
(b) ∆Hmixing
(c) ∆Vmixing
(d) All of these
Class 12 RBSE Chemistry Solutions Question 6.
On increasing temperature, vapour pressure of a substance
(a) always increases.
(b) decreases.
(c) does not depend on temperature.
(d) partially depends on temperature.
RBSE 12 Chemistry Solutions Question 7.
The osmotic pressure of 5% sugar solution will be
(a) 3.47 atm
(b) 5.07 atm
(c) 4.03 atm
(d) 2.09 atm
RBSE 12th Chemistry Solution Question 8.
On increasing temperature, the solubility of H2 gas in water
(a) increases
(b) decreases
(c) remains unchanged
(d) None of these
Answer:
1. (c), 2. (c), 3. (a), 4. (c), 5. (d), 6. (a), 7. (a), 8. (a)
RBSE Class 12 Chemistry Chapter 2 Very Short Answer Type Questions
Chemistry Class 12 RBSE Solutions Question 1.
Calculate molality of 10% (w/W) aqueous H2SO4
Answer:
Weight of solute (WB) = 10 g
Weight of solvent (Wa) = 100 – 10 = 90 g
Molecular weight (MB) = 98 g mol-1
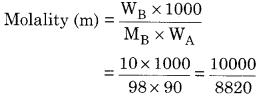
= 1.134 mol kg-1
RBSE Solution Class 12 Chemistry Question 2.
What is molarity. ? Write the effect of temperature on it.
Answer:
Molarity: It is the number of moles of solute dissolved per litre of the solution.
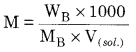
On increasing the temperature, molarity of solution decreases because on increasing temperature volume of solution increases .
12th RBSE Chemistry Solution Question 3.
Define the mole fraction of a substance in solution.
Answer:
Mole fraction-Mole fraction of a constituent (solute as well as solvent) is the fraction obtained by dividing number of moles of that constituent by the total number of moles of all the constituents present in the solution. In a binary mixture, there are two components A and B and their moles are nA and nB respectively. If their mole fractions are xA and xB
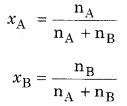
If the mass of A and B are WA and WB respectively and their molar masses are MA and MB then,
nA = \(\frac { { W }_{ A } }{ { M }_{ A } } \)
nB = \(\frac { { W }_{ B } }{ { M }_{ B } } \)
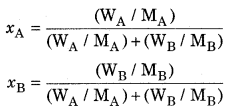
The sum of mole fractions of all the components are always equal to 1.
XA + xB = 1
RBSE Class 12th Chemistry Solution Question 4.
Does it advise to use ethylene glycol in radiators of cars in summer ?
Answer:
No, it is not advised to use ethylene glycol in radiators of cars in summer because ethylene glycol decreases the freezing point of water, prevents the freezing of water of radiator in winter, so it is advised to use ethylene glycol in winter.
Class 12th RBSE Chemistry Solution Question 5.
Define reverse osmosis ?
Answer:
If the pressure higher than the osmotic pressure is applied on the solution, the solvent will flow from the solution into the pure solvent through the semipermeable membrane. The process is called reverse osmosis.
RBSE Class 12 Chemistry Chapter 2 Short Answer Type Questions
RBSE Class 12 Chemistry Book Solution Question 1.
Explain the effect of temperature on solubility of solid in liquid. Give the formula of van’t Hoff factor which relates the abnormal molecular mass to normal molecular mass. How does it affect by the association and dissociation?
Answer:
Effect of temperature on solubility of solid in liquid–There is following equilibrium between soluble solid and solution in a saturated solution. Insoluble solid + Solute – Solvent Solution ⇌ Solution ;
∆Hsol = ± x k cal
According to Le-chatelier’s principle if ∆H > 0 (zero) i.e. on dissolving solute in solvent, energy is absorbed, hence on increasing temperature the solubility of solid solute increases .
Example: NH4 CI, KCI, AgNO3, NaNO3, KI etc.
If ∆H < 0 (zero) i.e. on dissolving solute in solvent energy is released, hence on increasing temperature the solubility of solid solute decreases.
Example: NaOH, Li2SO4, (CH3 COO)3 Ca etc.
van’t Hoff’s factor

If observed colligative property < calculated value or Mo > Mc (or i<1), there is association. If observed colligative property > calculated value or Mo < Mc (ori >1), there is dissociation.
RBSE Solutions For Class 12 Chemistry Chapter 1 Question 2.
The theoretical molecular mass and observed molecular mass of an ionic compound AB are 58.2 and 30 respectively. Calculate the van’t Hoff factor and degree of dissociation.
Answer:
Calculated molecular mass of AB = 58.2
Observed molecular mass of AB = 30
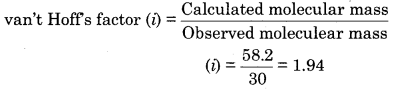
Degree of dissociation (α) = \(\frac { i-1 }{ n-1 }\)
Substance AB on dissociation gives 2 mol substance A and B. Hence,
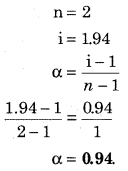
Chemistry Solutions Class 12 RBSE Question 3.
What are the differences between diffusion and osmosis ? Give an example of each. Represent the diffusion and osmosis by labelled digrams.
Answer:
Differences between diffusion and osmosis
| Osmesis | Diffusion |
| 1. In this process semi-permeable membrane is used. | 1. In this process no semi-permeable membrane is used. |
| 2. It takes place from lower concentration to higher concentration. | 2. It takes place from higher concentration to lower concentration. |
| 3. It takes place only in solutions. | 3. It takes place in gases as well as in solutions. |
| 4. It can be stopped or reversed by applying pressure on the solution with higher concentration. | 4. It cannot be stopped or reversed. |
Examples:
Diffusion:
When potassium permanganate is added in container of water then after some time violet colour spreads in container. This is an example of diffusion.
Representation of process of diffusion by digram:
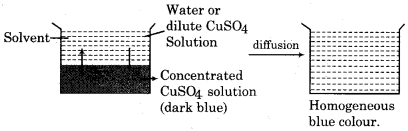
Osmosis:
If we put grapes in a concentrated solution of NaCl, the grapes shrink but if we put kismis or grapes into pure water, they swell. It is a phenomenon of osmosis.
Representation of process of osmosis by digram:
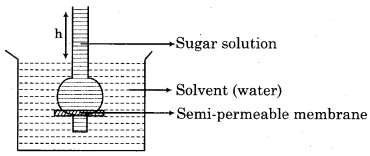
RBSE Solutions 12 Chemistry Question 4.
0.2L aqueous solution of protein contains 1.26 g protein. The osmotic pressure of this solution is 2. 57 x 10-3 bar at 300 K. Calculate the molecular mass of protein.
Answer:
Given that,
Volume ‘V’ = 0.2 L
Mass of solute ‘WB‘ = 1. 26 g
Temperature ‘T’ = 300 K,
Osmotic pressure ‘π’ = 2.57 x 10-3 bar
Gas constant ‘R’ = 0. 0821 L bar mol-1 K-1
![]()
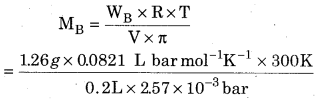
= 60377.04 g/mol
Molecular mass of protein = 60377.04 g/mol
RBSE Solutions For Class 12th Chemistry Question 5.
Prove that ∆Tb = Kb.m for solution having non-volatile solute.
Answer:
If boiling point of solution is Tb and the boiling point of solvent is \({ T }_{ b }^{ 0 }\) then,
Elevation in boiling point \(\Delta { T }_{ b }={ T }_{ b }-{ T }_{ b }^{ 0 }\)
Elevation in boiling point is directly proportional to lowering in osmotic pressure.
ΔΤb ∝ ΔΡ
According to Raoult’s law, lowering in vapour pressure is directly proportional to mole fraction of solute, hence,
ΔP α xb (x3 = mole fraction of solute)
Hence,
ΔΤb ∝ xB
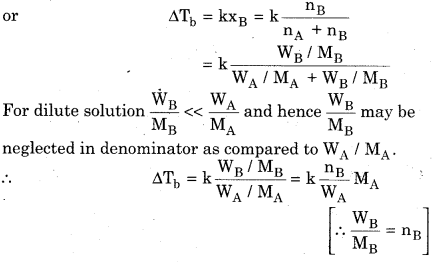
If WA is the mass of solvent in kg, then \(\frac { { n }_{ B } }{ { W }_{ A } } \) is equal to
molality (m) of the solution.
∆Tb = kMAm
Here k and MA are constants and hence their product
i.e., kMA is replaced by another constant kb.
∆Tb = Kbm
Where Kb is called boiling point-elevation constanr or molal elevation constant or molal ebullioscopic constant.
RBSE Solutions For Class 12 Chemistry Chapter 2 In Hindi Question 6.
How can the molecular mass of non-volatile substance determined from the component of vapour pressure ? Explain it.
Answer:
For non-volatile substance, relative lowering in vapour pressure of solution is equal to the mole faction of solute.
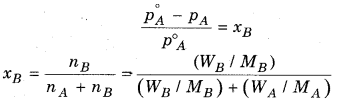
Where,
nB = Number of moles of solute
nA = Number of moles of solvent
WB = Mass of solute
WA = Mass of solvent
MB = Molecular mass of solute
MA = Molecular mass of solvent
According to. Raoult’s law
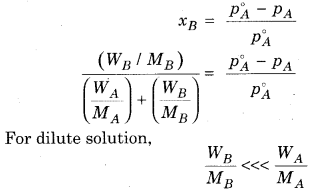
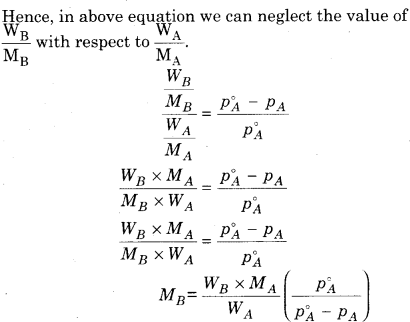
With the help of above equation we can calculate the value of molecular mass of non volatile solute, if all other factors in equation are known.
RBSE Solutions For Class 12 Chemistry In Hindi Question 7.
What do you understand by solubility of gases? Explain the factors affecting solubility of gases in liquid.
Answer.
Solubility of gases in liquids: All gases are less or more soluble in water or soluble in certain limit and form a true solution. The volume (in mL) of a gas which dissolved in 1 mL of water is called absorption coefficient.The solubility of gases in liquids is affected by the following factors:
- Nature of gas: The gases which react with solvent or ionized in solution are soluble in water. e.g. NH3, HCl(g) and SO2 are more soluble in water and form NH4OH, HCl(l) and H2SO4 compounds respectively.
- Nature of solvent: The polar gases are soluble in polar solvent while non-polar gases are soluble in non-polar solvents. It is like dissolves like rule e.g. HCl gas is more soluble in water than liquid benzene.
- Effect of Temperature: Temperature has a marked effect on the solubility of a solid in liquid. The solubility may increase or decrease with the rise in temperature depending upon the value of ∆sol H < 0. Let us recall, that saturated solution represents equilibrium between undissolved solute and dissolved solute.
Solute + Solvent ⇌ Solution; ∆sol H = ± x- If the value of ∆sol H < 0 i.e. the solution process is exothermic, then according to Le-chatelier’s principle, the increase of temperature will push the solution equilibrium in the backward direction. In other words, the solubility of solutes decreases with rise in temperature. Some examples are Li2SO4, Na2SO4 etc.
- If ∆sol H > 0, i.e. solution process is endothermic, then increase of temperature will push solution equilibrium in the forward direction. In other words, the solubility of such solutes increases with the rise in temperature. Some examples of such solutes are KCI, KNO3, NaNO3 etc.
- Effect of pressure: The solubility of gases is much affected by change in pressure. Henry proposed a relationship between pressure (P) and the composition of solution which is called Henry’s law. According to this law.
“At constant temperature, the amount of gas soluble in unit volume of solvent is directly proportional to pressure created by gas at equilibrium on the surface of solvent”.
If the mass of gas is m which is dissolved in unit volume 3 of solvent at pressure (P) then according to Henry’s law,
m ∝ P
m = kP
k = Henry constant
RBSE Solutions Class 12 Chemistry Question 8.
Calculate the temperature at which the solution of 54g glucose present in 250g water will be freezed.
(Kf =1.86 K kg mol-1)
Answer:
Given that:
Weight of solute (WB) = 54g
Molecular weight of solute (MB) = 180 g mol
Weight of solvent (WA) = 250g
Kf = 1.86 K kg mol-1
Depression in freezing point ∆Tf
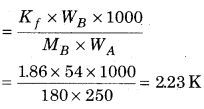
Depression in freezing point = 2.23 K
Freezing point of solution = ?
Freezing point of water = 273.15 K
(∆Tf) = Freezing point of water – Freezing point of solution
Freezing point of solution = 273.15 – 2.23= 270.92
Class 12th Chemistry RBSE Solutions Question 9.
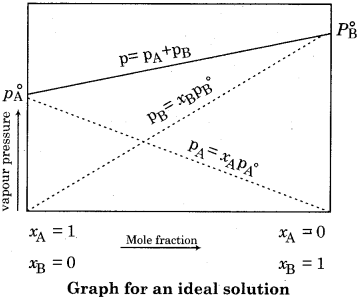
Draw a graph for an ideal solution made up by solute and solvent.
Answer: