Rajasthan Board RBSE Class 12 Biology Notes Chapter 5 Plant Water Relations
Introduction
- Survival of life is not possible without water.
- Water is one of the five basic elements considered in Indian mythology.
- Water acts as most suitable medium for all activities of life.
Important Properties of Water
- Universal solvent.
- Polar nature
- Hydrogen bonds
- High specific heat
- Highest density at 4°C
- Neutral pH (pH = 7)
- High cohesive and adhesive force
- Di-electric constant
- Availability in solid, liquid and gaseous form at limited temperature range.
![]()
Cell is the structural and function unit of body of all organisms
Different life activities are performed in the protoplasm of cell.
To understand the features of protoplasm and the biochemical reactions taking place in the protoplasm, it is necessary to know about the following
- Diffusion
- Permeability
- Osmosis
- Plasmolvsis.
- Imbibition etc.
Diffusion
When a bottle of ammonia is opened in a room it is due to diffusion only that the smell of ammonia spreads in entire room and when a crystal of copper sulphate is put in a glass of water entire water becomes blue coloured.
In a system of two or more substances, the direction and the rate of diffusion of molecules of the substances does not depend upon each other. For example when concentrated sugar solution and pure water are brought in contact, sugar molecules move towards water and water molecules move towards sugar solution.
Diffusion of molecules of different substances present in a system depends on their concentration and is not influenced by the presence of other substance, this is called independent diffusion.
Example : In plants diffusion of O2 , CO2 and water vapour is not influenced by the presence and concentration of the molecules of one another but is rather influenced by the concentration of the molecules of that particular substance in the atmosphere.
![]()
Factors Affecting Diffusion
(1) Temperature—Rate of diffusion is directly proportional to temperature, i.e. increase in temperature increases the kinetic energy of molecules, resulting into increase in rate of diffusion.
(2) Density : Rate of diffusion of a substance is inversely proportional to the square root of the density of the diffusing substance i.e. increase in the size of molecules reduces the rate of their diffusion. This means lesser is the size of molecules higher is the rate of their diffusion.
Rate of diffusion (r) =\(\frac{1}{\sqrt{\text { Density of molecules of the substance }(\mathrm{d})}}\)
Rate of diffusion = \(\frac{1}{\sqrt{\text { Size of molecules of diffusing substance. }}}\)
Hence diffusion of gas of high density is slower than the gas of low density.
(3) Pressure—Rate of diffusion is directly proportional to the pressure. Movement of molecules of any substance is always from the region of their higher diffusion pressure to the region of theirlower diffusion pressure.
In other words movement of molecules is from the region of their higher concentration to the region of their lower concentration.
(4) Medium—Rate of diffusion is slower through a dense medium as compared to a sparse medium.
![]()
Importance of Diffusion:
1. During photosynthesis and respiration exchange of gases (CO2 and O2) between plants and atmosphere takes place through diffusion.
2. During transpiration loss of water vapour from plant to the atmosphere is through diffusion.
3. Passive absorption of minerals to some extent occurs through diffusion.
4. Diffusion is also helpfull in translocation of food material (from leaves to roots) in plant body.
5. Osmosis and imbibition also involve diffusion.
6. Plant hormones are distributed in plant body by diffusion
Permeability
1. The rate by which a substance diffuses through a membrane (layer) is known as permeability of the membrane (layer).
2. Permeability is the specific property of the membrane (layer) due to which any substance is able to pass through it and enter the cell or comes out of the cell.
3. Any membrance (layer) across which molecules of both the solvent as well as the solute can freely move is called permeable.
4. Such a membrane across which solvent molecules can pass freely but which is completely impermeable to the solute molecules is called semi-permeable.
5. A membrane which is permeable to the solvent (water) molecules and molecules of only some solutes i.e. it is fully permeable to solvent (water) but shows permeability to molecules of selected solutes, is called selectively permeable of differentially permeable membrane. Example—Plasma membrane, and tonoplast.
6. A membrane (layer) across which both the solvent (water) as well as solute molecules can not pass through, is called impermeable. Example—Cork-cells, Cuticle.
Cell membrane or plasma membrane behaves both as semipermeable and selectively permeable in nature
Osmosis
Definition : Diffusion of only solvent molecules through semipermeable membrane is called osmosis. Osmosis can be defined as “movement of solvent molecules from the region of their higher concentration to the region of their lower concentration across a semipermeable or selectively permeable membrane is called osmosis.”.
- Osmosis is a special type of diffusion where a semipermeable membrane is necessarily present between two systems.
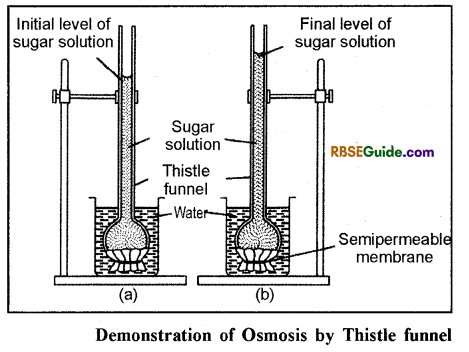
- The process can be understood and demostrated with the help of thistle funnel experiment.
- Parchment paper is properly tied over the broader face of the funnel.
- The tube of the funnel is then filled with sugar solution and its level is maked.
- The face of the funnel covered with parchment paper is kept in beaker filled with water as shown in fig. 5.1.
- After some time it is observed that level of sugar solution in the tube of the funnel rises up.
- According to the process of osmosis water molecules diffuse from the region of their higher concentrate (from beaker) to the region of their lower concentration (sugar solution in funnel) where as sugar molecules do not show diffusion because of the semipermeable nature of the parchment paper
Types of Osmosis
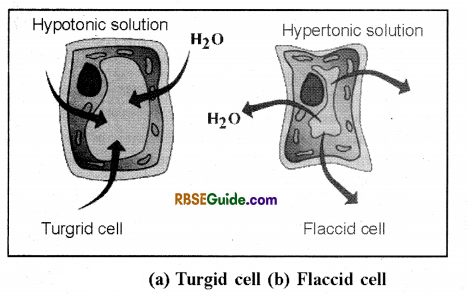
1. Endosmosis : When a normal plant cell is dipped in a hypotonic solution or pure water, it is observed that water molecules enter the plant cell. This is called endosmosis. In ward movement of water in any system through osmosis is called endosmosis.
Example :
- Swelling of raisins dipped in water.
- Entry of soil water in root hair cells.
2. Exosmosis : When a plant cell is dipped in a hypertonic solution (concentrated sugar solution) it is observed that water molecules begin to come out of cells. This is called exosmosis.
Example :
- Shrinking of grapes dipped in concentrated sugar solution.
- Adverse effect on crop plants of addition of excessive chemical fertilizer in soil.
![]()
Importance of Osmosis:
1. Root hair absorbs water from soil through osmosis.
2. Absorption of some dissolved minerals also takes place partly through osmosis.
3. turgidity of cells and growth of the young cells depend on osmosis.
4. Transportation of water from one part of the plant to other and cell to cell conduction occurs through osmosis.
5. Opening and closing of stomata is regulated by osmosis.
6. Turgid state of the cells is responsible for specific shape and form of leaf, flowers and fruits etc.
7. This process makes plant resistant against freezing and desiccation.
8. It is helpful in germination of seeds
Plasmolysis and Deplasmolysis
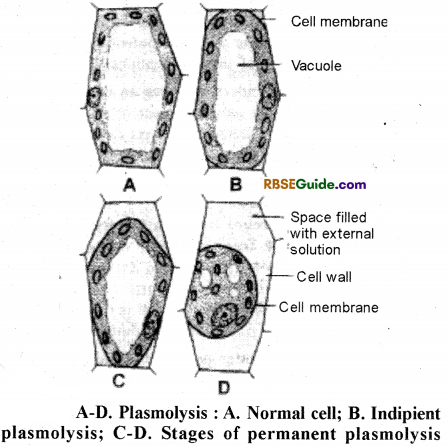
1. When a plant cell is dipped in a hypertonic solution, exosmosis takes place and the cytoplasm of the cell shows shrinking. This process is called plasmolysis.
2. When a normal plant cell is kept in hyptertonic solution, then due to exosmosis water from the cytoplasm and vacuole moves out of cell. Due to this the cytoplasm begins to shrink and as a result it separates from the cell wall and collects in comer of this cell. This state of the cell is reffered as plasmolysed cell and the process as plasmolysis.
3. The initial state, when the cytoplasm ju st begins to separate from the cell wall, is called incipient plasmolysis.
4. The cell in the incipient plasmolysis state when placed in a hypotonic solution, the cytoplasm regains its normal state. This process is called deplasmolysis. In this state the cytoplasm again streches against cell wall and the cell becomes turgid.
Demonstration of Plasmolysis
1. We take two peices of peeled out lower epidermis of leaf of Rhoeo discolor plant, and observe under microscope.
2. The epidermal cells show the vacuole filled with purple coloured sap.
3. One piece is put in water and the other in the sugar solution.
4. After some time these are seperately observed under microscope.
5. It is obsereved that the cells in the piece dipped in water are in turgid state and the purple coloured vacuole is in streched state.
6. The piece dipped in sugar solution shows shrinking of purple coloured contents of the cells which are collected in the centre or in a comer of the cell. This is plasmolysed state of the cell and demonstrates the process of plasmolysis.
7. The cells in plasmolysed state, when are placed in pure water, again become turgid and the purple coloured contents spread uniformly in the vacuole of the cell. This process is called deplasmolysis.
Different Types of Pressures Developed in Cell
1. Turgor Pressure (TP)
- In a living plant cell, the volume of the cytoplasmic contents increases due to endosmosis. As a result the plasma membrane streches against the cell wall.
- The cell wall is pressed outwards due to the pressure created by endosmosis. This pressure is called turgor pressure (TP).
- The value of turgor pressure in a flaccid cell is zero where as in a fully turgid cell the value of turgor pressure is maximum.
![]()
2. Wall pressure (WP)
- In a turgid cell, the cell wall creates a pressure on the cytoplasm and presses it inwards.
- This pressure created by the cell wall against turgor pressure is called wall pressure.
- In a fully turgid cell, the value of wall pressure becomes equal to the turgor pressure.
- Wall pressure acts in opposite direction of turgor pressure and thus prevents rupture of plant cell.
3. Osmotic Pressure (OP)
- The pressure which develops as a result of endosmosis and prevents further entry of water molecules from pure water to the solution separated by a semipermeable membrane is called Osmotic pressure.
- In other words, the maximum pressure developed in a solution as a result of endosmosis, which prevents further endosmosis in a solution separated from pure water by a semipermeable membrane is called osmotic pressure.
- Osmotic pressure of any solution is directly proportional to the concentration of the solute molecules in the solution.
- OP oc concentration of solute molecules in solution
- In a cell OP and TP both develop due to endosmosis.
- O.P. of pure water is zero.
- Water molecules move from the solution of low O.P. to the solution of high OP w’hen the tw o are separate by a semipermeable membrane.
Note : An apparatus used to measure osmotic pressure is called osmometer.
4. Diffusion Pressure Deficit
Diffusion pressure of pure water is maximum.
When some solute is mixed in pure water, then the diffusion pressure of the solution decreases in comparison to pure water. Hence there is always a difference in the diffusion pressure of pure water and of a solution.
This difference is due to the solute molecules. Greater is the amount of solute molecules in the solution, greater is the difference in diffusion pressure.
![]()
At normal temperature and atmospheric pressure, difference between the diffusion pressure of the solution and diffusion pressure of pure water is called diffusion pressure deficit (DPD) of the solution. DPD = Diffusion pressure of pure water – Diffusion pressure of soluiton.
Diffusion pressure deficit is also called as suction pressure (SP) because it is indicative of the capacity of the solution to draw solvent molecules from pure solvent separated by the semipermeable membrane.
In other words DPD represents water demand of the solution in a cell.
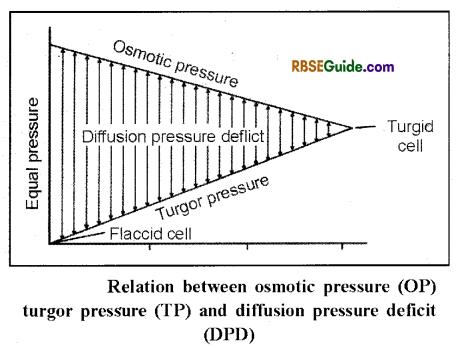
In a cell, the value of diffusion pressure deficit is equal to the difference between the osmotic pressure of the solution and the turgor pressure. The relation between these can be expressed as follows :
DPD = OP – TP
(i) In a flaccid cell, the value of TP is zero. At this stage the DPD becomes equal to the osmotic pressure
DPD = OP TP (if TP = O)
then DPD = OP
(ii) Now when a cell is dipped in hypotonic solution or pure water, endosmosis results in to development of turgor pressure and the DPD of the cell begins to decrease. Endosmosis continues till the value of TP becomes equal to OP and cell becomes fully turgid.
(iii) In a fully turgid cell value of DPD becomes zero because TP becomes equal to OP
DPD = OP – TP The value of
DPD = OP – TP Because OP = TP
= OP – OP = zero.
Hypothesis of Water Potential
1. Hypothesis of water potential was proposed by Slatyer and Taylor (1960).
2. According to laws of thermodynamics each component of any system has some free energy which allows it to work.
3. During osmosis water molecules move across the scmipermeable membrane and for this free energy is required.
4. Wien a cell is kept in pure water, there exist a difference of free energy between molecules of pure water and the free energy of water molecules present in the solution.
5. This difference of free nergy between the pure water and water molecules of the solution is called water potential.
6. In other words, the value of water potential of a solution is equal to the difference in the value of free enegy of solvent molecules of the solution and free energy of molecules of pure solvent.
7. It is expressed by Greek symbol F(psi) and its unit is Bar or atm.
8. It is not possible to determine absolute value of water potential and hence its value is taken as zero.
9. Freen energy of pure water (solvent) is maximum and when some solute is added to it, the free energy of solvent (water) decreases.
10. Hence free energy of any solution is less than that of pure water meaning by always negative.
According to laws of thermodynamics
![]()
11. DPD of a solution is equal to the water potential but value of DPD is positive and that of water potential is negative.
12. OP is called solute potential or osmotic potential and it is expressed as Fs.
The value of osmotic pressure and the osmotic potential is equal but the only difference is that value of osmotic pressure is positive and that of osmotic potential is negative. Its value is also negative.
13. TP is called pressure potential and it is expressed as ‘Fp. Value of pressure potential is positive.
According to this hypothesis relationship between.water potential, osmotic potential and pressure potential is as follows:
Ψw = Ψs + Ψp.
Where Ψw = Water potential
Ψs = Osmotic Potential
Ψp = Pressure potential
here value of
Ψw is negative
Ψs is negative.
Ψp is positive
so
Ψw = Ψs + Ψp.
Concept of an Osmotic System
- Concentrated sugar solution is filled in a bag made up of semipenneable membrane
- It is kept in a beaker containing dilute sugar solution.
- Water molecules from beaker begin to move to the concentrated solution in the bag through semipermeable membrane.
- After sometime, the bag streches due to endosmosis.
- When the bag becomes fully streched it creates a pressure on the solution to prevent further entry of water. This pressure is called turgor pressure.
- The effect of turgor pressure on water potential is called pressure potential.
In a plant cell the cell wall is reffered as matrix.
1. In a fully turgid plant cell the pressure developed by the cell wall is called matrix potential. It is represented as Ψm.
2. Although in soil the matrix potential is important but it is neglighible in cellular osmotic system.
Hence
Water potential (Ψw) = Ψm + Ψs + Ψp
As the value of Ym is negligible in cellular system
Ψw = Ψs + Ψp
3. Since the value Ψ s is negative and that of Ψ p is positive. So in a fully turgid cell when the value of Ψ p becomes equal to Ψ s, further endosmosis stops and Ψw becomes zero.
Imbibition
1. Definition : Absorption of water molecules by solid or colloidal substances without forming solution is called imbibition. In fact this kind of absorption is called adsorption.
2. In imbibition colloidal substances and hydrophilic solid substances draw water. The substances which imbibe water are called imbibants.
3. Imbibants are hydrophilic colloids and can imbibe large amount of water by surface attraction.
4. The primary and secondary wall layers of plant cells are made up of cellulose, pectin and lignin etc. These are hydrophilic in nature and can adsorb large amount of water.
5. The rate of imbibition is affected by temperature, nature of imbibant, and difference in water potential.
![]()
6. Imbibition can occur only when there is affinity between the imbibant and water.
7. A considerable force may develop due to imbibition within the plant body. This is called imbibition pressure.
8. In imbibition water always moves with some force from saturated region to drier region.
Importance of Imbibition
- In germinating seeds, seed coat burst due to imbibition pressure.
- It also plays important role (together with osmosis) in the intake of soil water by root-hairs.
- It is believed to be an important force involved in ascent of sap.
- In many plants resurrection is on account of presence of hydrophilic colloids.
