Rajasthan Board RBSE Class 9 Science Notes Chapter 4 Chemical Bond and Chemical Equation
Symbol
- In 1873 Berzelius gave a system to represent the element by the symbol. A symbol is the shortest form of the name of an element. Symbol of an element is the first letter or another letter of the English name or Latin name. Usually three methods are used in this system.
- First letter of the name of the element is used as symbol, e.g.
Name of the element Symbol Hydrogen H Oxygen 0 Nitrogen N Carbon C Phosphorous P Sulphur S Flourine F Uranium U Vanadium V Boron B Iodine I
Name of two or more elements begin with the same letter. In such cases, one of the element is given first letter of its name as symbol. While the other elements are given two letters of the English name or Latin name .
In two letters symbol, the first letter is the capital letter but the second letter is the small letter. For example:
| Name of the element | Symbol | Letter selected |
| Carbon | C | (First letter of name) |
| Calcium | Ca | (First and second letter) |
| Chlorine | Cl | (First and third letter) |
| Cadmium | Cd | (First and third letter) |
| Chromium | Cr | (First and third letter) |
| Cesium | Cs | (First and third letter) |
| Cobalt | Co | (First and second letter) |
In some cases, the symbols are derived from Latin names of the elements.
| English name | Latin name | Symbol | |
| 1. | Silver | Argentum | Ag |
| 2. | Gold | Aurum | Au |
| 3. | Copper | Cuprum | Cu |
| 4. | Iron | Ferrum | Fe |
| 5. | Mercury | Hydrargyrum | Hg |
| 6. | Potassium | Kalium | K |
| 7. | Sodium | Natrium | Na |
| 8. | Lead | Plumbum | Pb |
| 9. | Tin | Stannum | Sn |
General rules are given for the name of elements above atomic number 100. The rules are as follows. Their name is given according to IUPAC (International Union of Pure and Applied Chemistry) system.
- The symbol of the element is represented by three letters.
- Name of the element is written according to order, with the last digit of the name, ium is added as suffix.
For example:Atomic number Name according to IUPAC Symbol 101 Un-nil-unium Unu 102 Un-nil-bium Unb 103 Un-nil-trium Unt 104 Un-nil-quadium Unq 105 Un-nil-pentium Unp 106 Un-nil-hexium Unh 107 Un-nil-septium Uns 108 Un-nil-octium Uno 109 Un-nil-enium Une
Ion
- We know that smallest and undivisible particle of an element is called atom. An atom has nucleus. Nucleus contains positively charged particles protons and electrically neutral neutrons. Negatively charged electrons revolve around the nucleus in different orbits. Number of electrons and protons are equal, so atom is electrically neutral.
- Each element has definite atomic number and electronic configuration. Chemical properties of elements depend on electronic configuration of atoms. When an atom loses electron, it becomes positively charged and when it gains electron, it becomes negatively charged. In short, ion is an electrically charged atom (or group of atoms). A positively charged ion is known as cation. A negatively charged ion is known as anion.
- Cation: A cation is formed by the loss of one or more electrons by an atom.
There is need of energy to form cation. For example:
A(g) + energy → A+ (g) + e–
A+(g) + energy → A2+ (g) + e– - Metal atoms form monovalent, bivalent, trivalent and polyvalent cations.
For example:
Monovalent Cations: Hydrogen, H+; Sodium Na+; Potassium K+
Bivalent Cations: Copper, Cu2+ ; Barium, Ba2+; Strontium ; Sr2+
Trivalent Cations: Aluminium, Al3+; Boron B3+; Arsenic As3+
Polyvalent Cations: Manganese, Mn7+; Mn 4+; ChromiumCr4+ ; Cr5+ Tin, Sn2+; Sn4+ - Anion: An anion is formed by the gain of one or more electrons by an atom. Energy is liberated while forming anion
X(g) + e- → X–(g)+ energy
Non – metals form monovalent, bivalent, trivalent etc. anions.
Monovalent anions: Chloride Cl–, Flouride F–, Bromide Br2-, Iodide1-
Bivalent anions: Oxide O2-, Sulphide S2-
Trivalent anion: Nitride N3-, Phosphide P3-
One or more atoms combine to form polyatomic ion
Polyatomic cation: Example : Ammonium (NH4)+
Polyatomic anion: Example : Nitrate (NO3)–, Hydroxide (OH)–
Carbonate (CO3)2-, Phosphate (PO4)3-. A group of more than two atoms from polyatomic ions. - Size of ions: The metal atoms lose electrons to form cations (positively charged ions). A cation is smaller than the atom from which it is formed. This is because when a neutral atom loses electrons, the number of electrons will be less than protons. As a result, the attraction between positively charged nucleus and electrons will increase, so the shell of electrons contract and the size of cation will be smaller.
- Anions are formed when non-metal atoms gain electrons. An anion is bigger than the atom from which it is formed. This is because, when a neutral atom accepts electrons, the number of electrons will be more than protons. As a result, repulsion between electrons will increase, so the shell of electrons will expand and the size of an anion will be bigger.
Radical
- Acids, bases and salts dissociate into two types of ions having opposite charge when dissolved in water i.e. ionization occurs in the aqueous solution. Cation is called positive radical and anion is called negative radical. For example, in aqueous solution sodium chloride (NaCl) dissociates into sodium ions (Na+)and chloride ions (Cl–).
- Radical is a charged group of atoms of one or more elements which behave and take part in chemical reactions as a single unit without splitting, but do not remain free for long time.
Radicals are of two types:
- Basic radicals and
- Acid radicals. .
- Basic radicals are positively charged and written first, followed by acid radicals which are negatively charged.
- Basic radicals:
Ag+1 , Na+1, Ba+2, Zn+2, Ca+2, Mg+2, Cu+2, Sb+3, As+3, Pb+2, Bi+3, Cr+3, Fe+3, etc. - Acid radicals:
Charge on the radicals also show their valencies
Cl-1, Br-1, l-1 ,(NO2)-1, O–2, S–2, (SO4)–2, (CO3)–2, (PO4)–2, (CH3COO)-1 etc.
Valency
The electrons present in the outermost shell of an atom are known as the valence electrons. They govern the chemical properties of atoms. The atoms of elements having completely filled outermost shell, which has eight electrons show little chemical activity, i.e., high stability. Such elements are called inert elements. Of these inert elements, the helium atom has two electrons in its outermost shell and all the other elements have atoms with eight electrons In the outermost shell.
The number of electrons lost or gained or shared by an atom to become stable or to achieve an octet electronic configuration known as valency, of that element. In other words, it is the combining capacity of the atom of an element with the atom(s) of other element(s) in order to complete its octet.
Variable valency:
• Some elements have more than one value of valency.
For example, variable valencies of some elements are as follows:
| Copper(Cu) | +1, +2 | Mercury(Hg) | +1. +2 |
| Iron (Fe) | +2, +3 | Tin(Sn) | +2, +4 |
| Gold (Au) | +1, +3 | Silver (Ag) | +1, +2 |
| Arsenic(As) | +3, +5 | Antimony (Sb) | +3, +5 |
Molecular Formula
A molecular formula represents the composition of a molecule of the substance in terms of symbols of the elements present in the molecule e.g., (1) Molecular formula of sodium chloride is NaCl, (2) Molecular formula of acetic acid is C2H4O2.
Significance of the formula of a substance: A formula gives the following information:
- Formula represents the name of the substance. For example, NaCl is the name of sodium chloride .
- Formula represents one molecule of the substance. NaCl represents one molecule of sodium chloride.
- Formula gives the name of all constituent elements present in the molecule. For example, sodium (Na) and chlorine(Cl) are constituent elements of NaCl. Constituent elements of Na2SO4 are sodium (Na), sulfur (S) and oxygen (O).
- Formula gives information about the number of atoms of elements present in one molecule, e.g, Na2SO4 tells that there are two atoms of sodium (Na), one atom of sulfur (S) and four atoms of Oxygen (O).
- If atomic weights are known, the molecular weight of the substance can be found. Atomic weight of sodium is 23 and that of chlorine is 35.5. Therefore, molecular weight of sodium chloride (NaCl) is 23 + 35.5 = 58.5.
- Atomic weights of sodium, sulfur and oxygen are 23, 32 and 16 respectively, so the molecular weight of sodium sulfate (Na2S04) is 23 × 2 + 32 + 16 × 4 = 46 + 32 + 64 = 142.
- The number written in the beginning of a formula denotes the number of molecules. For example, in the formula CuSO4 5H2O of copper sulphate molecule there are 5 molecules of water (H2O).
Method of writing a formula:
Write symbol of positive radical and along with the negative radical.
- Bismuth: Bi
- Carbonate: CO3
- Magnesium: Mg
- Oxide: O
Afterwards their valency should be written at the top (above). If necessary, radicals may be written in brackets.
- +3: Bi
- +2: CO3
- +2: Mg
- -2: O
Now shift the valencies crosswise to lower right of the radicals. If both are same you need not write, just simplify, if they can be simplified. If a radical receives number more than one, enclose it within brackets. Do not enclose the atom within brackets.
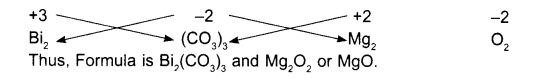
Examples of compound with their molecular formula:
Hydrogen Sulfide:
- Symbols
- Charges
- Formula
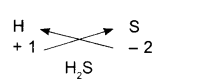
Note: When the subscript is number 1, subscript is not written.
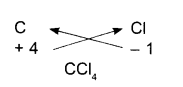
Carbon Tetra chloride:
- Symbols
- Charges
- Formula
Magnesium Chloride:
- Symbols
- Charges
- Formula
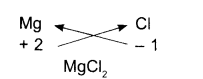
In other words, the positive and negative charges must balance each other and the overall formula must be neutral
For example:
Calcium oxide:
- Symbols
- Charges
- Formula
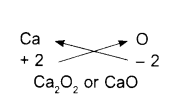
Note: When the valency of both elements are numerically equal, the subscripts are also not written.
Aluminium oxide:
- Symbols
- Charges
- Formula
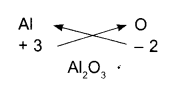
Sodium nitrate:
- Symbols
- Charges
- Formula
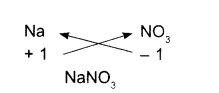
Potassium carbonate
- Symbols
- Charges
- Formula
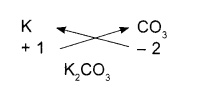
We use brackets when we have two or more of the same ions in the formulae.
For example:
Aluminium hydroxide:
- Symbols
- Charges
- Formula
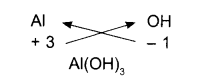
Ammonium sulfate
- Symbols
- Charges
- Formula
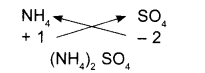
All subscripts must be reduced to lowest term (except for molecule or covalent compound)
For example:
Tin (IV) oxide
- Symbols
- Charges
- Formula
Chemical Bond
When atoms of an element or atoms of different elements combine to form a molecule, a force of attraction develops between the participating atoms, which holds them together. This force of attraction between two similar or different atoms in a molecule is called chemical bond
Inert Gas configuration is stable:
Inert gases do not react chemically either with one another or with other elements because there are eight (8) electrons in their outer most (valence) shell.
Distribution of electrons in some of the inert gases are:
Ne (10) = (Neon) (2,8)
Ar (18) = (Argon) (2,8,8)
Kr (36) = (Krypton) (2,8,18,8)
Distribution of electrons in fluorine, sodium and calcium atoms are:

Types of Chemical Bonds: There are two types of chemical bonds:
- Ionic bond or Electrovalent bond
- Covalent bond
Concept of Ionic and Covalent Bond:
The exchange of valence electrons between atoms gives rise to a chemical bond. In case, electrons are transferred from the valence shell of one atom to the valence shell of another atom, the bond formed is called ionic bond or electro covalent bond. In case, when electrons are mutually shared by atoms, the bond so formed is called covalent bond
Ionic bond or Electrovalent bond:
The bond is formed between electropositive element and electronegative element. When an atom of electropositive element comes close to an atom of negatively charged element, the electropositive atom aquires the configuration of nearest inert gas by losing electrons from the outermost shell and becomes positively charged. The electronegative element acquires the configuration of nearest inert gas by taking electron in its outermost shell and becomes negatively charged.
These opposite charged ions are held together by the electrostatic force of attraction. This bonding is called ionic bond or electrovalent bond and the compound, so formed is called ionic compound or electrovalent compound. The number of electrons which an atom of an element donates or accepts is called its electrovalency.
For example: When sodium atom reacts with chlorine atom, the sodium atom loses one electron from its outer most shell and this electron goes in the outermost shell of chlorine. Both make two ions Na+ and Ch having electronic configuration of two inert gases. Due to opposite charges, sodium ions and chloride ions are held together by electrostatic force is of attraction to form sodium chloride (NaCl) molecule.
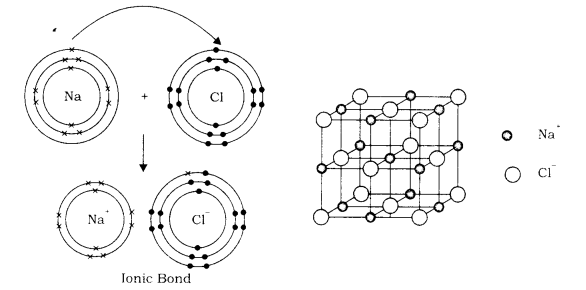
Properties of ionic compounds
- Physical State: They are generally solids.
- Structure of crystal: Different ionic compounds form different crystal lattice.
- High melting point and boiling point: Ionic compounds generally have high melting and boiling points. The reason is that charged ions present in ionic crystal are bound by strong electrostatic force. As lot of energy is required to break strong electrostatic force, so ionic compounds have high melting and boiling points. ,
- Solubility: Ionic compounds are mainly soluble in polar solvents (e.g. water) and are insoluble in non-polar liquids (such as benzene and carbon tetrachloride etc.)
- Brittle nature: Ionic compounds are brittle. If force is applied on them, ionic layers slip over one another, ions with similar charge come closer. Repulsion between them breaks the compound, and thus, ionic compounds are brittle in nature.
- Hardness: Ionic compounds are generally relatively hard, because cations and anions are held firmly by strong electrostatic forces.
- Ionization reaction: The ionic compounds break into ions in water. So, the ionic reactions are fast, due to opposite charged ions
- Conduction of electric current: Ionic compounds in solid state are bad conductor of electric current, but in fused (molten) state or in solution they are good conductors. In molten state the electrostatic forces holding the oppositely charged ions weaken considerably. Electrons of free ions conduct the electric current .
Covalent bond
This kind of bond is generally formed by non metallic elements having seven, six, five or four electron, in outermost shell. The only exception is hydrogen, which has only one electron. The chemical bond formed by sharing of electrons between two atoms is known as covalent bond. The sharing of electrons takes place in such a way that each atom in the molecule gets the stable electron arrangement of nearest inert gas.
Examples:
- Formation of Chlorine molecule: Chlorine molecule is formed, when bonding takes place between two atoms of chlorine. These atoms have seven electrons each in their outermost shell. In order to have stable configuration, they need one electron each. Two chlorine atoms share one electron pair mutually and attain stable configuration of argon having 8 electrons in outermost shell. This is shown by the following diagram:
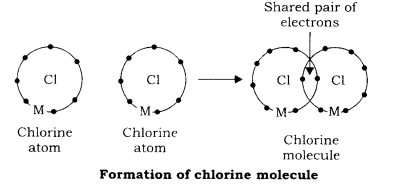
- Formation of chlorine molecule
Formation of water molecule (H2O):
Two atoms of hydrogen and one atom of oxygen take part in the formation of water molecule. Hydrogen atom has one valence electron, so it needs one more electron to have a stable configuration like that of helium gas. Oxygen has six electrons in its outer most shell. Oxygen atom needs two more electrons to have stable configuration like that of neon (2.8). - So, one atom of oxygen shares two electrons with two hydrogen atoms to form a water molecule, as shown in the following figure:
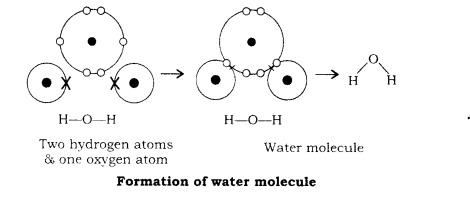
Properties of Covalent compounds:
- Physical state: Covalent compounds are generally gaseous or volatile liquids. Some covalent compounds are also solids, such as diamond and graphite.
- Melting and boiling points: Covalent compounds generally have low melting and boiling points.
- Reason: The molecules of covalent compound are held together by weak forces. Thus, very small amount of energy is required to break bonds between two or more molecules, so they have low melting points and boiling points.
- Electric conductivity: Covalent compounds are bad conductor of electricity, i.e., they do not conduct electricity.
- Reason: It is because covalent compounds do not have free electrons. Hence, they do not conduct electricity.
- Solubility: Covalent compounds are insoluble in water (polar solvent). But they are soluble in non-polar solvents (organic solvents) such as benzene, carbon tetra chloride, alcohol, petrol etc.
- Reactions of covalent compounds go on with slow rate.
- Most of covalent compounds have density less than that of water.
- Covalent bonds are directional. Covalent molecules have specific geometrical shape.
Chemical equation:
- The method of representing a chemical reaction with the help of symbols and formula of the substance involved in it, is
- known as a chemical equation.
For example, the chemical equation: Zn + 2HCl → ZnCl2 + H2 - The above equation tells us that zinc metal reacts with dilute hydrochloric acid to form zinc chloride and hydrogen gas.
- Atomic weight of Zn = 65, H = 1, O = 16 and Cl = 35.5 Molecular weight of hydrochloric acid (2HCl) = 2(1+35.5) = 73 Molecular weight of zinc chloride (ZnCl2) = 65 + 35.5 * 2 = 65 + 71 = 136 and, molecular weight of H2 = 1 * 2 = 2.
- The above equation tells us that 65 gm of zinc reacts with 73 gm of hydrochloric acid as a result of which 136 gm of zinc chloride and 2 gm of Hydrogen are produced.
Characteristics of an equation:
- Chemical equation is a symbolic representation of chemical reaction.
- It gives information about the reactants and products in a chemical change. In the above equation, zinc and hydrochloric acid are reactants, while zinc chloride and hydrogen are products.
- Knowing the atomic weights and using balanced equation, the molecular weights of the reactants and products can be determined.
- If the reactants or products or both are gases we know that a gram molecular weight of a gas occupies 22.4 litre volume at NTP. In above reaction, the gas hydrogen shall occupy 22.4 litres.
Limitations of a chemical equation:
There are certain facts which are not represented in the chemical equation.
- Chemical equation does not tell always about the physical states of reactants and products.
- Chemical equation does not give idea about conditions, i.e., temperature, pressure and presence of a catalyst.
- It does not give any idea about the rate of the reaction, whether it is slow or fast.
- It does not give any idea about the concentration of reactants and products.
- The chemical equation fails to give any information, whether heat is evolved or absorbed in the chemical reaction.
- The chemical equation does not always give indication about the nature of reaction, whether it is reversible or irreversible reaction.
- A chemical equation fails to give any idea about the mechanism of the reaction.
- A chemical equation does not tell about the precautions which are to be taken, while carrying out the reaction.
To make chemical equations more informative:
- There are four physical states for the products and reactants of a chemical reaction. Solid is indicated by the symbol (s), liquid state by the symbol (/), gas by the symbol (g) and aq is written for aqueous solution.
- In the equation an arrow (↑) pointing upwards is used to indicate a gaseous product, and an arrow (↓) pointing downwards indicates a precipitate.
Zn + H2SO4 → ZnSO4 + H2 ↑
BaCl2 + 2AgNO3 → 2AgCl + Ba(NO3)2 ↓ - To indicate the concentration of a reactant or product, (dil) is written for dilute solutions and (cone.) for concentrated solutions, below their formula
CaCO3 + 2HCl → CaCl2 + H2O + CO2 ↓ - The conditions of reaction such as temperature, heat, pressure and catalyst are written above or below the arrow.
For example:
\(\mathrm{N}_{2}(g)+3 \mathrm{H}_{2}(g) \frac{450^{\circ} \mathrm{C}, 200 \mathrm{atm}}{\rightleftharpoons \mathrm{e}+\mathrm{Mo} \text { Catalst }} 2 \mathrm{NH}_{3}(g)+22400\) - Colour is written below the reactants and products.
- Those reactions in which heat is evolved are known as exothermic reactions. The quantity of heat evolved is written on the product side with + (plus) sign, such as
\(\mathrm{N}_{2}+3 \mathrm{H}_{2} \stackrel{455^{\circ} \mathrm{C}, 2,00 \mathrm{atm}}{\longrightarrow} \cdot 2 \mathrm{NH}_{2}+22400\) - Those reactions in which heat is absorbed are called endothermic reactions. The quantity of heat absorbed is written on products side with – (minus) sign, such as
\(\mathrm{N}_{2}+\mathrm{O}_{2} \longrightarrow 2 \mathrm{NO}-43200\) calories - If a reactant is heated then A sign is put on the arrow, such as
\(2 \mathrm{KClO}_{2} \stackrel{\Delta}{\longrightarrow} 2 \mathrm{KCl}+3 \mathrm{O}_{2} \uparrow \)