Rajasthan Board RBSE Class 12 Biology Notes Chapter 11 Respiration
Introduction
- All living organisms require energy for performing various life activities. This energy is obtained from food materials. The food materials are synthesized by green plants.
- Solar energy is stored in the food materials by green plants in the process of photosynthesis with the help of chlorop lasts.
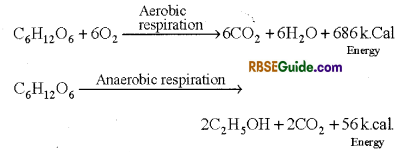
- The energy stored in the complex organic compounds is released by the process of oxidation. In this process (Oxidation) complex organic compounds are broken down to CO2 and water in the presence or absence of oxygen and energy is released. This process can be expressed by the following reactions.
Definition
- Respiration is the process taking place in all living cells in which high energy containing complex organic compounds are broken down to low energy containing simple substance with release of energy. Normally carbon dioxide is released and oxygen is used in the process and potential energy is converted into kinetic energy.
- This energy is used in all activities taking place in cells. Respiration is basically a vital process which occurs in all living cells at all times.
- Most animals and plants use O2 and release CO2 during respiration.
![]()
Respiratory Substrates
- The high energy containing organic compounds which undergo oxidation during respiration reactions and release energy during the process are called respiratory substrates.
- These are stored in the form of carbohydrates, fats or protein molecules. Of these, carbohydrates are the primary respiratory substrates.
- Hexose sugar is the first carbohydrate used as respiratory substrate during respiration.
- In the absence of carbohydrates, fats are used as respiratory substrates, and when carbohydrates fats are consumed, oxidation of proteins begins.
- Blackman used the term floating respiration for respiration by oxidation of carbohydrates and protoplasmic respiration for oxidation of proteins. Protoplasmic respiration normally takes place during starvation and sickness.
Types of Respiration
Respiration is of two types :
- Aerobic respiration
- Anaerobic respiration
- Aerobic Respiration
This type of respiration takes place in the presence of oxygen i.e. O2 is used in the process and food material is completely oxidized to release CO2, H2O and energy.
In all animals and plants this is the normal method of respiration. Aerobic respiration can be represented by following chemical equation.

Anaerobic Respiration
- This type of respiration take place without the use of oxygen. The complex organic compound is incompletely oxidized. In this process ethyl alcohol or organic acid and CO2 are formed and very small amount of energy is released.
- This is also called intramolecular respiration. Anaerobic respiration can be represented by following chemical equation.
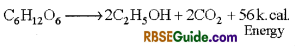
Stored seeds, germinating seeds, and fleshy fruits temporarily respire anaerobically. Several fungi and bacteria regularly respire anaerobically.
![]()
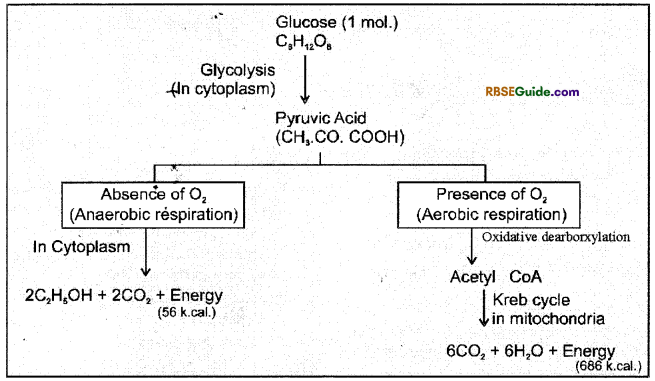
Relation between Aerobic and Anaerobic respiration
Difference between Aerobic and Anaerobic Respiration
| S.No. | Aerobic Respiration | Anaerobic Respiration |
| 1. | Oxygen (O2) is used. | Oxygen (O2) is not used. |
| 2. | Normally takes place in all living cells of all animals and plants. | Takes place in some fungi, bacteria and stored and germinating seeds. |
| 3. | 38 ATP molecules are formed by complete oxidation of one molecule of Glucose. | Glucose molecule is not oxidized completely and it’s in complete oxidation yields only 2 molecules of ATP. |
| 4. | In this respiration, reactions of Glycolysis take place in cytoplasm and Krebs cycle reactions take place in mitochondria. | All reactions take place in cytoplasm. |
| 5. | Substrate molecule is completely oxidised. | Substrate molecule is incompletely (partially) oxidised. |
| 6. | End products are CO2, H2O and energy. | End products are Alcohol or Organic acid, CO2 and small amount of energy. |
Site of Respiration
1. In eukaryotic organisms which normally perform aerobic respiration, the main site of respiration is mitochondria. Two important steps of aerobic respiration i.e. Krebs cycle and electron transport system take place in mitochondria.
2. Mitochondria are double membrane cell organelles. Both the outer and inner membranes are made up of lipids and proteins.
3. The space between two membranes (40-70A) is called perimembrane space.
4. The outer membrane is smooth whereas the inner membrane is folded in the form of several irregular projections in the space of mitochondria. These foldings or projections are called cristae (singular-crista). The inner space of cristae is called intra cristae space. On the surface of cristae, several stalked granules remain attached. These are called primary particles (F1 particles) or oxysomes. ATP formation takes place on these particles through electron transport system.
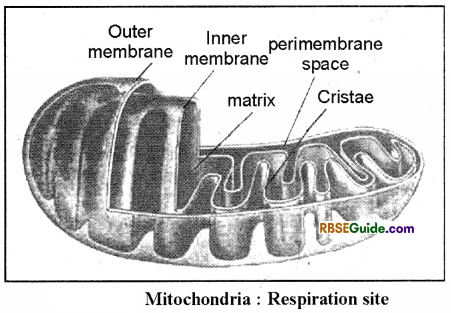
5. The internal space of mitochondria is called as matrix. It consists of semifluid proteinaceous material. Several enzymes and coenzymes (NAD, NADP, ADP), electron carriers, ribosomes, RNA and DNA are included in the matrix.
6. Mitochondria are also called power-hosue of cell, because energy currency is formed in the form of ATP molecules in the mitochondria during electron transport system.
Mechanism of Aerobic Respiration
- Carbohydrate is the main respiratory substrate in the process of respiration, and it begins with Glucose.
- In the absence of carbohydrates, fats and proteins are used.
- The initial reactions both in aerobic and anaerobic respiration, take place in the cytoplasm of cell. In these reactions one molecule of Glucose is broken down to two molecules of Pyruvic acid. This process is called glycolysis or EMP path. Oxygen is not required in this process.
- Pyruvic acid formed in glycolysis enters into mitochondria and forms Acetyle coenzyme A (Acetyl CoA).
- In mitochondria it is further broken down into CO2 and H2O through Krebs cycle reactions and energy is released. This complete break down of Glucose takes place in the presence of O2.
![]()
The complete mechanism of aerobic respiration can be divided into three steps.
- Glycolysis
- Kreb’s cycle (Tricarboxylic acid cycle, TCA)
- Electron Transport System (ETS).
Glycolysis
1. The term Glycolysis has originated from Greek work “Glycose”, (meaning sugar) and Lysis (meaning break down, dissolve, analysis), and means breakdown of sugar. This is a complex bio-chemical process and is completed in ten steps. Different steps of Glycolysis were worked out by G Embden, Otto Meyerhoff, and Parnas in 1930, hence this process is also called EMP path. This is completed in cytoplasm of the cell and O2 is not used in the process.
2. Glycolysis takes place by the same reactions in all types of organisms and is found in both aerobic and anaerobic respiration.
3. It can be defined as, “Breakdown of glucose molecule in to pyruvic acid through a series of biochemical reactions along with release of energy is called glycolysis”.
4. All the 10 biochemical reactions occuring during glycolysis can be studied under three heads.
- Phosphorylation of Glucose
- Splitting of phosphorylated hexose molecule in two molecules of phosphoglyceraldehyde.
- Formation of two molecules of pyruvic acid.
Phosphorylation of Glucose
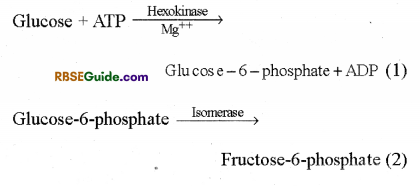
1. In the first step of glycolysis one molecule of glucose changes in glueose-6-phosphate by use of one ATP molecule in the presence of hexokinase In the presence of isomerase enzyme, Glucose-6-phosphate changes into fructose-6-phosphate. Fructose-6-phosphate using one molecule of ATP further changes into fructose 6-diphosphate in the presence of enzyme The reactions taking place during phosphorylation of glucose are as follows :

Splitting of Phosphorylated Hexose molecule in two molecules of Phosphoglyceraldehyde
1. In this reaction fructose 1-6-diphosphate breaks down in one molecule of phosphoglyceraldehyde (3-PGAL) and one molecule of dihydroxyacetoiie phosphate (DHAP) in the presence of aldolase enzyme.
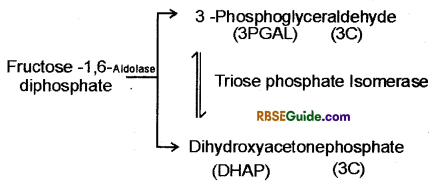
These two compounds are interconvertible in the presence of triphosphate isomerase enzyme. Of these compounds oxidation takes place of only 3-PGAL. Hence as 3-PGAL is oxidised, DIIAP keeps on changing to 3-PGAL.
Formation of two molecules of Pyruvic Acid
3-phos-phoglyceraldehyde (3PGAL) molecules are oxidized and converted into two molecules of pyruvic acid by the following reactions.
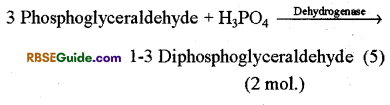
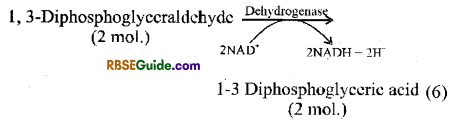
(i) Formation of 1-3 diphosphoglyceric acid : 3-PGAL, molecule reacts with H3PO4 in the presence of enzyme dehydrogenase and forms 1, -3 diphosphoglyceraldehyde. This is oxidized to form 13 diphosphoglyceric acid. This is called oxidative step. Hydrogen ions released in this reaction reduce NAD+ to NADH + FP which generates ATP through electron transport chain in case of aerobic respiration.
(ii) Formation of 3-phosphoglyceric acid from 1-3 diphosphoglyceric acid : In this reaction one phosphate group is removed from 1-3 diphosphoglyceric acid in the presence of enzyme diphosphoglycerokinase. ADP is converted in ATP and 3-phosphoglyceric acid is formed.
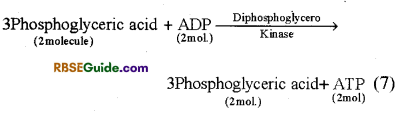
![]()
(iii) Isomerisation of 3-phosphoglyceric acid into 2 phosphoglyceric acid : 3-phosophglyceric acid is changed to its isomeric form 2-phosphoglyceric acid in the presence of enzyme phosphoglyceromutase.

(iv) Formation of 2-phosphoenol pyruvate from 2- phosphoglyceric acid : 2-phosphoglyceric acid changes to 2 phosphoenol pyruvate by losing one molecule of water in the presence of enzyme enolase.
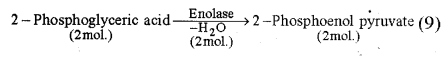
(v) Formation of Pyruvic acid from 2-phosphoenol pyruvate : In the presence of enzyme pyruvic kinase, phosphoenol pyruvate loses one phosphate and forms pyruvic acid and ATP molecule.
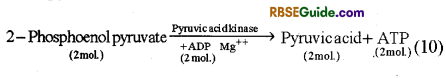
The process of Glycolysis can be explained in totality by the following reaction.

Glycolysis
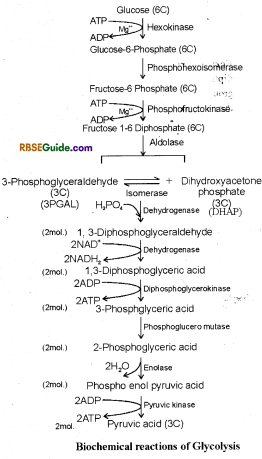
Summary of Glycolysis
1. One molecule of Glucose (6C) breaks down to form two molecules of pyruvic acid (3C)
2. Four ATP molecules are generated in substrate level phosphorylation of which two molecules are used, hence there is net gain of 2 molecules.
3. 2 molecules of 1-3 diphosphoglyceric acid are formed by oxidation of 2 molecules of 1-3 diphosphoglyceraldehyde. 2 molecules of NADH + H+ are generated in this reaction.
4. Each NADH + H+ generates 3 ATP molecules through electron transport system. Thus 6 molecules of ATP are formed, provided glycolysis is followed by Krebs cycle.In reactions of glycolysis O2 is not used and CO2is not released.
5. Some intermediate compounds of glycolysis are used in other synthetic processes. Hence it is also called catabolic-resynthesis or oxidative anabolism. Ex. PGAL is used in synthesis of glycerol. Similarly phosphoglyceric acid is used in formation of several amino acids, such as Glycine, Serine, Cystein etc.
![]()
Fate of Pyruvic Acid
Pyruvic acid is the end product of glycolysis (EMP Path) On the basis of availability or absence of oxygen further breakdown of pyruvic acid may take place by following paths :
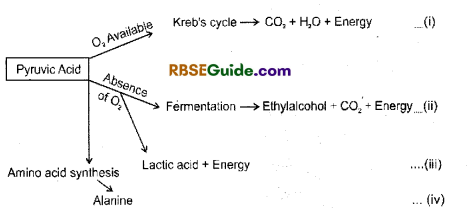
Aerobic oxidation of Pyruvic Acid
1. The second phase of respiration begins, when pyruvic acid formed in the cytoplasm enters mitochondria. In the matrix of mitochondria one carbon atom of pyruvic acid is oxidised in the form of CO2.
2. This process is called oxidative decarboxylation. After this it is oxidized in the presence of pyruvic dehydrogenase enzyme and combines with Co-enzyme – A (Co A) to form acetyl coenzyme A(Acetyle CoA).
3. This process requires five co-factors and these are CoA, NAD+, Mg++, Lipoic acid (LA) and Thymine pyrrophosphate (TPP).
4. The process of formation of acetyl coenzyme A connects glycolysis and Krebs cycle and hence it is called Link reaction or Gateway reaction. It can be represented by following equation :
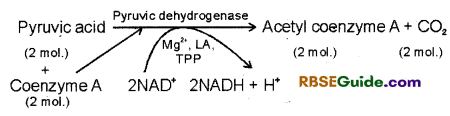
In this way,2molecules of pyruvic acid are oxidized to form two molecules of acetyl 90 enzyme A and 2 molecules of NADH + FT. These 2 molecules of NADH + If forms 6 ATP molecules through ETS.
![]()
Krebs Cycle, Citric Acid Cycle, Tricarboxylic Acid (TCA) Cycle
1. These reactions occuring in mitochondria were discovered by British Bio-chemist Sir H.A. Krebs in 1937. He was awarded with Noble Prize for this work in 1953.
2. Krebs cycle reactions begins with the formation of citric acid and hence it is also called Citric acid cycle. Because citric acid contains three acidic groups (-COOH), hence this cycle is also named as Tri carboxylic acid (TCA) cycle.
3. In the begining of Krebs cycle, 2 carbon containing compound acetyl coenzyme-Atransfers it’s carbon atoms to oxaloacetic acid and 6 carbon compound-citric-acid is formed.
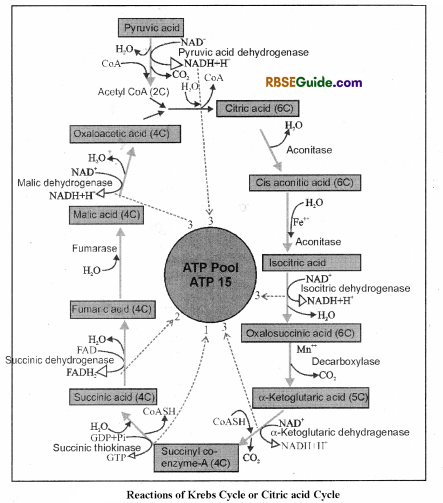
Main steps of Krebs cycle can be explained as follows:
1. Formation of Citric Acid : In mitochondria, 2 carbon containing acetyl coenzyme A reacts with 4 carbon compound oxaloacetic acid in the presence of condensing enzyme citrate synthase and forms citric acid by combining with water. Coeznyme-A is set free in this reaction.

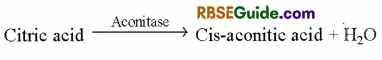
2. Formation of Cis-aconitic acid : Citric acid loses one molecule of water and changes in to cis-aconitic acid in the presence of aconitase enzyme.
3. Formation of Isocitric acid : Cis-aconitic acid reacts with water in the presence of enzyme aconitase and changes in to isocitric acid.
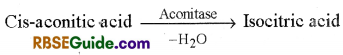
4. Formation of Oxalosuccinic acid : In this reaction isocitric acid is oxidised in the presence of enzyme, dehydrogenase and forms oxalosuccinic acid. NAD+ is reduced to NADH – H+ in this reaction.
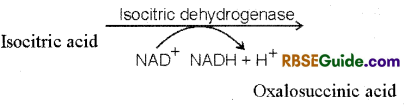
5. Formation of a-Ketoglutaric acid : Oxalosuccinic acid loses one molecule of CO2 imthe presence of enzyme decarboxylase and forms a-ketoglutaric acid. In Krebs cycle, this is the only 5 carbon containing acid.
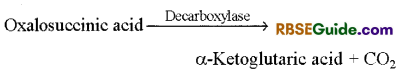
6. Formation of Succinyl coenzyme A : 5 carbon compound a-ketoglutaric acid undergoes oxidative decarboxylation in the presence of enzyme dehydrogenase and forms succinyl coenzyme-A. In this reaction NAD+ is reduced to NADH + H+ and CO2 is given out.
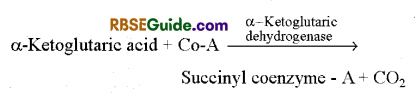
7. Formation of Succinic acid : In the presence of thiokinase enzyme succinyl coenzyme-A changes to succinic acid by hydrolysis. Coenzvme-A is set free in the reaction. Energy is stored as GTP which later on forms ATP.
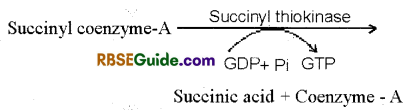
8. Formation of Fumaric acid : Succinic acid is oxidised to fumaric acid in the presence of succinic dehydrogenase enzyme. Hydrogen atom released in the process reduces FAD+ to FADH2.
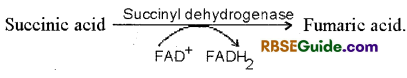
9. Formation of Malic acid : Fumaric acid combines with water in the presence of Fumarase enzyme and forms malic acid.

10. Formation of Oxalo acetic acid : In the last reaction of Krebs cycle, malic acid is oxidised and forms, oxaloacetic acid in the presence of malic dehydrogenase. In this reaction NAD+ is reduced to NADH + H+
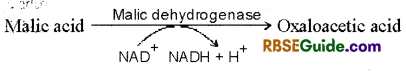
Oxaloacetic acid again condenses with acetyl coenzym-A and enters in Krebs cycle.
![]()
In this way, by complete oxidation of 1 (one) molecule of pyruvic acid 3 molecules of CO2 are released. Because 2 molecules of pyruvic acid are formed by 1 molecule of glucose (glycolysis), in complete oxidation of 1 molecule of glucose 6 molecules of CO2 are formed.
Summary of Krebs Cycle
All reactions of Krebs cycle can be summarised in the form of following reaction and fig. (11.4).
Acetyl coeznyme-A + 3H2O + 3NAD+ + FAD+ + GDP + Pi →3 CO2 + 3 NADH + IE + FADIF, + GTP + Acetyl CoA
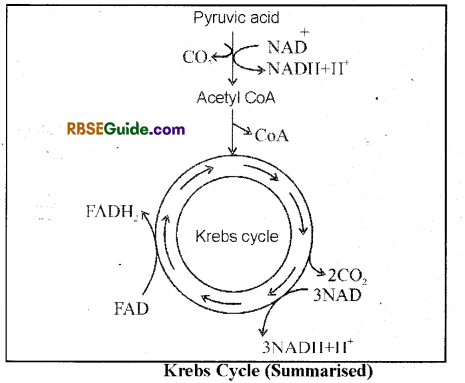
Importance of Krebs cycle
(a) ATP molecules-formed in Krebs cycle reactions are used as source of energy in various activities of life.
Intermediate compounds formed during Krebs cycle are used in the synthesis of several biomolecules. Ex. succinyl coenzyme-A acts as precursor molecule for chlorophyll synthesis and a-ketoglutaric acid, oxaloacetic acid and pyruvic acid are used in synthesis of amino acids.
Electron Transport System
1. For continuity of rea ctions of glycolysis and Krebs cycle, it isnecessary that NADH + H+ and FADH2 formed during these reactions are oxidized back to NAD+ and FAD+.
2. This oxidation occurs in mitochondria through electron transport system and energy is released in the form of ATP.
3. During electron transport system electrons flow from one electron acceptor to another in a definite sequence.
4. The electrons jump from high energy level acceptor to the low energy level acceptor releasing energy at some steps which is used in ATP synthesis.
5. All enzymes participating in these reactions are found in the inner membrane of mitochondria.
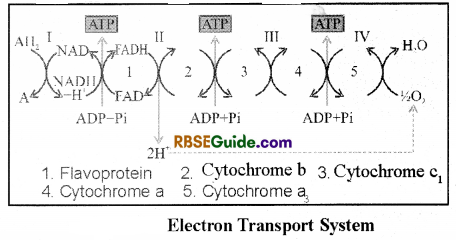
![]()
The components of electron transport system (ETS) or electron carriers have been sequenced as follows, which are called complexes.
| S.No. | Name of complex | Components of complex |
| 1. | Complex I | FMN, Fe-S |
| 2. | Complex II | Fe-S |
| -s | Complex III | Cytochrome b-Cyt. ct |
| 4. | Complex IV | Cyt. a and Cyt. a3 |
| 5. | Complex V | ATP synthetase |
1. In addition to these, two electron carriers cytochrome c and coenzyme Q (Ubiquinone) are also included in the sequence.
2. NADH + H+ and FADH2 are oxidized and the electrons are transported in a definite sequence through ETS. This can be explained as follows :
3. NADH + H+ formed in the matrix of mitochondria during Krebs cycle are oxidized to NAD+ by dehdrogenase enzyme. The electrons released in this reaction are accept ed by components of complex I found on the inner membrane. This multiprotein complex mainly includes NADH, ubiquinone oxidio-reductase, and flavin mono nucleotide (FMN).
4. The components of complex II receive electrons released in oxidation of succinic acid in presence of succinate ubiquinone oxidoreductase enzyme. The reducing equivalents are transferred through FADH2
5. Electrons from the reduced ubiquinone are transported to the components of complex IE i.e. cytochrome b and cyt. The cytochrome cx is a small protein loosely attached to the surface of inner membrane of mitochondria. It serves as a mobile carrier and transports electrons from complex HI to Complex TV.
6. Cytochrome c-oxidase complex (Cyt. a, Cyt a3) constitutes the last carrier in the chain of electron transport.
7. It is reffered as terminal oxidase of the cytochrome chain.
8. The reduced cyhtochrome O3 has the ability to transfer electrons to O2 and reducing it to H2
![]()
9. In essence, the ETS is a chain of carriers where electrons transported from complex I to complex (TV) flow from a higher to a lower level of energy. During this, ATP synthatase complex convert AJ)P and inorganic phosphate into ATP.
10. The number of ATP molecules formed during this process depends on the nature of electron donor.
11. One molecule of NADH + TP generates 3 ATP whereas one molecule of FADH2 generates only 2ATP.
12. Oxygen (O2) acts as the terminal acceptor of electrons in this process.
13. As in this process phosphorylation takes place in the presence of oxygen by its utilization in oxidation, it is called as oxidative phosphorylation.
Chemiosmotic Theory of ATP Synthesis
1. Cellular reactions such as photosynthesis and respiration involve energy conversion in the form of ATP. This energy is utilised in various biochemical reactions. ATP is known as universal currency of cell. ATP is formed by oxidative phosphorylation in respiration arid by photophosphorylation in photosynthesis
2. Peter Mitchell (1961) propounded chemiosmotic theory for ATP synthesis. This theory explains the mechanism of ATP synthesis both in chloroplast and mitochondria. According to this theory, transport of positive charged protons (Hydrogen ions) across the membranes of mitochondria, chloroplasts and bacteria takes place through the enzymes involved in respiration and photosynthesis via components of electron transport chain. Due to this, electrochemical proton gradient is created on two sides of membrane. This electroproton gradient has two features :
3. Difference in concetration of hydrogen ions or difference in pH on the two sides of the membrane.
4. Difference in the electrochemical proton gradient on two sides of the membrane.
5. These two create a proton motive force due to which proton (H+) move and enter due to concentration gradient and ATP synthesis takes place from ADP and inorganic phosphate with the help of ATPase enzyme.
Anaerobic Breakdown of Pyruvic acid
1. Pyruvic acid, the end product of glycolysis can be oxidized both in the presence and absence of oxygen. Its complete oxidation in the presence of oxygen releases CO2, H2O and high amount of energy. In the absence of oxygen pyruvic acid is incompletely oxidized and this is called anaerobic respiration.
2. In this process, first acetaldehyde is formed and CO2 is released by decarboxylation or fermentation. Acetaldehyde is reduced and forms alcohol. NADH + H+ is oxidized to NAD+. These two reactions are catalyzed by decarboxylase and dehydrogenase enzymes.
Fermentation
1. Anaerobic respiration is synonymous with fermentation. Fermentation is a process found in most bacteria and fungi which takes place in the absence of O2 or is perfomed without using O2. In fermentation sugar is incompletely oxidized and alcohol or organic acids are formed with release of CO2.
Pasteur (1857) proved that alcoholic fermentation is the result of metabolic activity of yeast cells. Buchner (1897) isolated zymase enzyme from yeast cells. This enzyme is capable of bringing about fermentation even in the absence of yeast cells.
![]()
Types of Fermentation :
On the basis of the end product formed, fermentation can be of following types :
Alcoholic Fermentation
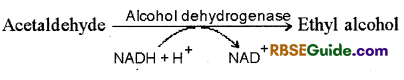
1. This process is found in yeast, some other fungi and some higher plants. This is a form of commonly found anaerobic respiration. Pyruvic acid, formed as end product of glycolysis, forms alcohol through following two steps :
2. It the first step decarboxylation of pyruvic acid results in the formation of acetaldehyde ancfrelease of CO2.

In the second setp in the presence of enzyme dehdyrogenase and NADH + H+, acetaldehyde is reduced to form alcohol & NAD+
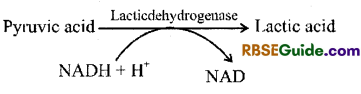
Lactic acid Fermentation
1. This process is found in many bacteria (.Lactobacillus, Clostridium) and muscle cells of Human. In this process pyruvic acid is reduced to lactic acid in the presence of enzyme lactic dehydrogenase and NADH + H+.
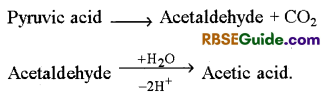
Acetic Acid Fermentaiton
1. This process is found in Acetobacter aceti bacterium. In this process pyruvic acid first forms acetaldehyde which then changes into acetic acid.
.
Butyric Acid Fermentation
This process is found in Bacillus butyricum and Clostridium butyricum bacteria. In this, pyruvic acid first forms acetoacetic acid which then forms butyric acid.
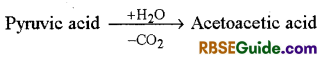
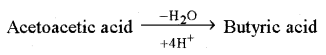
Difference Between Respiration and Fermentation
| S.No. | Respiration | Fermentation |
| 1. | This takes place in the presence of O2 | It does not require presence of CO2. |
| 2. | This is found in all living cells. | Living cells are not necessary for this. |
| 3. | Glucose is completely oxidized and forms CO2 and H2O | Glucose is incompletely broken down and forms alcohol or organic acid and CO2. Water is not formed. |
| 4. | Energy is released in high amount. | Energy is released in very low amount as heat. |
Other Pathways of Glucose Breakdown
1. Hexose Monophosphate Pathyway HMP or Pentose Phosphate Pathway (PPP)
2. Normally aerobic respiration is completed through glycolysis and Krebs cycle reactions. But in some organisms there exists an alternative path for complete oxidation of glucose which is called pentose phosphate path.
3. In this process hexose sugar is completely oxidized through involvement of a five carbon containing sugar hence it is called pentose phosphate path.
4. This was first worked out by Warberg and Dickons (1938) in animal tissues.
5. Racker and co-workers (1954) studied in details different reactions taking place in this process. This process is completed in the cytoplasm of cells in presence of oxygen. The process can be explained by following steps :
Phosphorylation of Glucose molecule : Glucose molecule is phosphorylated in the presence of ATP by enzyme hexokinase.
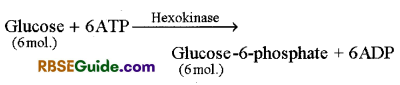
Oxidation of Glucose- 6- phosphate : Glucose – 6- phosphate is oxidized and forms phosphogluconic acid by enzyme glucose-6-phosphate dehydrogenase
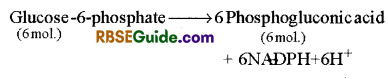
Oxidative decarboxylation ofPhosphogluconic acid: 6- Phosphgluconic acid is converted in to ribulose – 5 phosphate by oxidative decarboxylation in the presence of enzyme 6-phosphogluconic dehydrogenase. NADP+ is reduced to NADPH + H+ in this reaction.
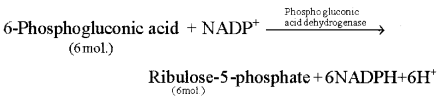
- In the subsequent reactions, ribulose-5 phosphate is converted in to several phosphorylated intermediate compounds through a series of metabolic interconvertible reactions.
- The ultimate result is complete oxidation of glucose phosphate with release of 6 molecules of CO2.
- For release of each molecule of CO2 in these reactions one molecule of NADPH + FT is formed. 12 molecules of NADPH + H+ formed in the process are oxidized through electron transport system and generate 36 molecules of ATP. Ribulose-5 phosphate formed as an intermediate in this cycle (HMP), is used in the synthesis of different compounds such as DNA, RNA, ATP, FAD. Coenzyme A etc.
Entner Doudoroff Pathway
- This is found in some bacteria. This shows oxidation of sugar into pyruvic acid. The intermediates formed during this path are different from those formed during normal glycolysis. This pathway was first studied in bacterium named as Pseudomonas saccharophila.
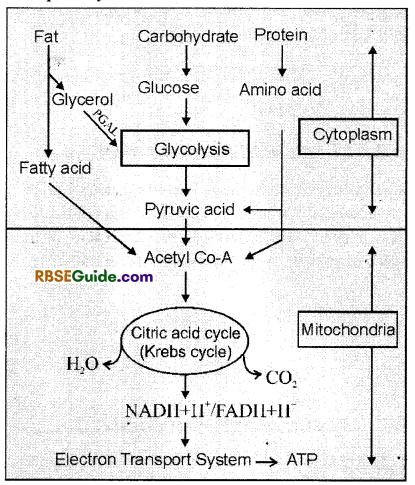
Inter-relationship Between Respiratory Substrates
1. Carbohydrates are normally used first as main respiratory substrate by all living organisms, but some organisms may use proteins, fats and organic acids also as respiratory substrate under specific conditions.
2. When fats are used as respiratory substrate, these are first broken down to fatty acids and glycerol.
3. The fatty acids enter Krebs cycle in the form of acetyl coenzyme-A. Glycerol first changes in to phosphoglyceraldehyde (PGAL) and then enters glycolysis.
4. In case proteins act as respiratory substrate, these are broken down to aminoacids by protease enzyme. The amino acids enter reactions of respiration along with pyruvic acid.
![]()
5. Carbohydrates are the first respiratory substrate after which fats are used and then organic acids. Proteins act as repiratory substrate in the end.
Respiration is an amphibolic process :
- The biochemical reactions in which complex organic compounds are broken down to simple compounds are known as catabolic activities and those in which synthesis of organic compounds takes place are called anabolic activities. During respiratory path both catabolic as well as anabolic activities take place, hence it is called amphibolic process.
Respiratory Quotient
During the process of respiration different organic compounds are oxidized and normally oxygen is used and carbon dioxide is released. The ratio of the volume of CO2 released to the volume of O2 used in respiration is called respiratory quotient (R.Q.) it is measured with the help of Ganong’s respirometer.
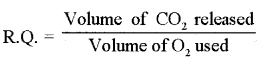
The value of R.Q. is helpful in determining the type of substrate used and type of respiration. The value of R.Q. varies with the type of substrate used in respiration. This can be explained by following :
(i) R.Q. of carbohydrates : When carbohydrate is respiratory substrate and it is completely oxidized, its R.Q. is always unit or 1 (one). In this process, the volume of CO2 released is always equal to the volume of O2 used.

(ii) R. Q. of fats: In oil seeds (Mustard, Ground nut, Cotton seeds etc.) fats are used as respiratory substrate during germination. In fat molecules the amount of O2 is less in comparison to carbohydrates. Hence fats require more oxygen for their oxidation and so value of R.Q. of fats is always less than one.
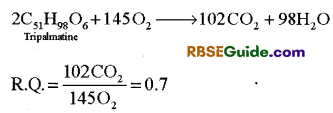
(iii) R.Q. of proteins: Protein molecules also have amount of Q2 less in comparison to amount of carbon like fats. Hence oxidation of proteins also requires more O2 and hence value of R.Q. of proteins is also less than one (<1), (0.7-0.9).
Proteins act as respiratory substrate in the absence of carbohydrates and fats. Ex. In Human proteins are used as respiratory substrate after prolonged fasting and its continuance is indicative of death of Human.
(iv) R.Q. of organic acids : In some plants organic acids act as respiratory substrates. The molecules of organic acids have amount of oxygen more than carbon. Hence oxidation of organic acids requires much less oxgyen and release CO2 in large amount. Hence value of R.Q. of organic acids is always more than one (>1) (4.0)
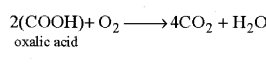
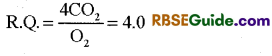
Respiratory Quotient of citric acid nad malic acid is 1.14 and 1.33 respectively.
(v) R.Q. of succulent or fleshy plants : In fleshy or succulent plants such as Opuntia etc. although carbohydrate is used as respiratory substrate but it is not completely oxidized. As a result intermediate compounds are formed but C02 is not released. Hence in these plants, the value of R.Q. is zero.
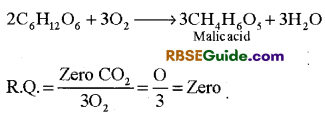
(vi) R.Q. in Anaerobic Respiration : In anaerobic respiration CO2 is released but O2 is not used hence in this type of respiration the value of R.Q. = oc
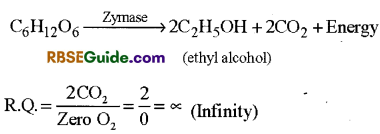
It is observed that lesser is the value of R.Q. more is the energy released in the process. Hence more energy is released from one molecule of fat where as energy released in oxidation of organic acid and during anaerobic respiration is much less.
Importance of Respiration
- The energy released during respiration is used by plant in different metabolic activities.
- During this process several intermediate compounds are formed and these are necessary for metabolic activities of cell.
- CO2 releasd in this process maintains gaseous balance in the atmosphere.
- Complex, insoluble organic compounds are converted into simple soluble compounds.
- The potential energy stored in food is converted into kinetic energy.
Factors Affecting Respiration
Rate of respiration is affected by several factors. Rate of respiration is highest in the actively dividing meristematic cells of plant. The factors affecting rate of respiration can be divided into two.
- External factors
- Internal factors
(1) External or Environmental Factors
Tern perature: It is the most important factor affecting rate of respiration. Normally rate of respiration increases to a certain limit by increase in temperature from 5.0°C to 30.0°C. In this range, the rate of respiration increases as per Vont Hoff’s rule. According to this a rise in temperature by 10°C, rate of respiration becomes double.
Increase in temperature beyond 35.0°C results in to decrease in rate as the enzymes begin to denaturalize. At very low temperatures also enzymes become inactive and rate of respiration decreases. In cold storage the fruits and vegetables escape spoilage because of this.
2. Oxygen : Oxygen is necessary for aerobic respiration because it acts as terminal acceptor of electrons. At low concentration of oxygen both aerobic and anaerobic respiration may take place, but when concentration of oxygen becomes zero only anaerobic respiration takes place. Under such a condition value of R.Q. becomes infinite (qo).
3. Water: Water acts as a medium for all the metabolic activities. The protoplasm contains upto 90-95% water. Water plays important role in enzyme activation, gaseous diffusion, transportation etc.
In dry seeds and fruits rate of respiration is extremely low due to shortage of water and so these can be stored for long duration. In presence of water stored carbohydrates convert into soluble sugar and rate of respiration increases.
![]()
4. Light : Rate of respiration is not influenced directly by light and so it takes place in the presence as well as absence of light. However light as a factor may influence rate of respiration as follows :
- Light causes increase in temperature which may cause increase in rate of respiration.
- Photosynthesis results in the synthesis of sugar which acts as respiratory substrate.
- In presence of light stomata open and so gaseous exchange is facilitated.
5. Carbon dixoide (CO2) : Increase in concentration of CO2 inhibits respiration. Hence it has adverse effect on germination of seeds and rate of growth of plants. Heath experimentally proved that increase in concentration of CO2 causes closure of stomata and rate of respiration decreases due to shortage of oxygen.
Internal or Plant Factors :
1. Protoplasm : Meristematic cells have dense and active cytoplasm and so rate of respiration is relatively high in these cells as compared to the mature cells. Activity of protoplasm is affected by several factors such as hydration, pH, temperature etc.
2. Respiratory substrate : Different types of sugars such as glucose, fructose, maltose etc. present in the cell are quickly used in respiration. In comparison to these starch and fats need to be first converted in to soluble form and only then they can be used as respiratory substrate. This is the reason why in hospitals a patient is administered directly with, glucose, where as the normal diet of healthy persons contains starch and fats.
3. Age of Cells : Rate of respiration is much higher in the young cells as compared to mature and old cells. Wound and Injured part: Rate of respiration sharply increases in the wound and injured part to promote healing.
RBSE Class 12 Biology Notes
